Sequence effects of aminofluorene-modified DNA duplexes: thermodynamic and circular dichroism properties
- PMID: 16449208
- PMCID: PMC1356535
- DOI: 10.1093/nar/gkj480
Sequence effects of aminofluorene-modified DNA duplexes: thermodynamic and circular dichroism properties
Erratum in
- Nucleic Acids Res. 2006;34(6):1945
Abstract
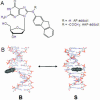
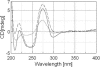
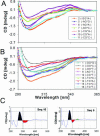
Circular dichroism (CD) and UV-melting experiments were conducted with 16 oligodeoxynucleotides modified by the carcinogen 2-aminofluorene, whose sequence around the lesion was varied systematically [d(CTTCTNG[AF]NCCTC), N = G, A, C, T], to gain insight into the factors that determine the equilibrium between base-displaced stacked (S) and external B-type (B) duplex conformers. Differing stabilities among the duplexes can be attributed to different populations of S and B conformers. The AF modification always resulted in sequence-dependent thermal (T(m)) and thermodynamic (-DeltaG degrees ) destabilization. The population of B-type conformers derived from eight selected duplexes (i.e. -AG*N- and -CG*N-) was inversely proportional to the -DeltaG degrees and T(m) values, which highlights the importance of carcinogen/base stacking in duplex stabilization even in the face of disrupted Watson-Crick base pairing in S-conformation. CD studies showed that the extent of the adduct-induced negative ellipticities in the 290-350 nm range is correlated linearly with -DeltaG degrees and T(m), but inversely with the population of B-type conformations. Taken together, these results revealed a unique interplay between the extent of carcinogenic interaction with neighboring base pairs and the thermodynamic properties of the AF-modified duplexes. The sequence-dependent S/B heterogeneities have important implications in understanding how arylamine-DNA adducts are recognized in nucleotide excision repair.
Figures

References
-
- Heflich R.H., Neft R.E. Genetic toxicity of 2-acetylaminofluorene, 2-aminofluorene and some of their metabolites and model metabolites. Mutat. Res. 1994;318:73–114. - PubMed
-
- Beland F.A., Kadlubar F.F. Metabolic activation and DNA adducts of aromatic amines and nitroaromatic hydrocarbons. In: Cooper C.S., Grover P.L., editors. Handbook of Experimental Pharmacology. Heidelberg: Springer-Verlag; 1990. pp. 267–325.
-
- Gillet L.C., Scharer O.D. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem. Rev. 2006 DOI: 10.1021/cr040483f, http://dx.doi.org/10.1021/cr040483f - DOI - PubMed
-
- Cui X.S., Eriksson L.C., Moller L. Formation and persistence of DNA adducts during and after a long-term administration of 2-nitrofluorene. Mutat Res. 1999;442:9–18. - PubMed

