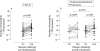Adding salmeterol to an inhaled corticosteroid: long term effects on bronchial inflammation in asthma
- PMID: 16449264
- PMCID: PMC2104614
- DOI: 10.1136/thx.2005.051292
Adding salmeterol to an inhaled corticosteroid: long term effects on bronchial inflammation in asthma
Abstract
Background: Addition of the long acting beta2 agonist salmeterol to inhaled corticosteroids leads to better symptomatic asthma control than increasing the dose of inhaled corticosteroids. However, little is known about the long term effects of adding salmeterol on the asthmatic inflammatory process, control of which is considered important for the long term outcome of asthma.
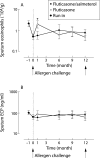
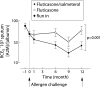
Methods: After a 4 week fluticasone run-in period, 54 patients with allergic asthma were randomised to receive twice daily treatment with fluticasone 250 microg with or without salmeterol 50 microg for 1 year in a double blind, parallel group design (total daily dose of fluticasone 500 microg in both treatment groups). Primary outcomes were sputum eosinophil numbers and eosinophil cationic protein concentrations. Secondary outcomes were neutrophil associated sputum parameters and a respiratory membrane permeability marker. The effects on allergen induced changes were determined before and at the end of the treatment period.
Results: Adding salmeterol to fluticasone resulted in improved peak expiratory flow, symptom scores, rescue medication usage, and bronchial hyperresponsiveness (p < 0.05 for all). There was no sustained effect on sputum cell differential counts and cytokine concentrations during the treatment period or on changes induced by allergen challenge at the end of treatment (p > 0.05). However, adding salmeterol significantly reduced sputum ratios of alpha2-macroglobulin and albumin during the treatment period (p = 0.001).
Conclusions: The addition of salmeterol to fluticasone produces no sustained effect on allergen induced cellular bronchial inflammation but leads to a significant improvement in size selectivity of plasma protein permeation across the respiratory membrane. This may contribute to the improved clinical outcome seen in patients with allergic asthma when a long acting beta2 agonist is combined with inhaled corticosteroids.
Conflict of interest statement
The Department of Pulmonology and Laboratory of Experimental Immunology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands receive research grants from GlaxoSmithKline, Numico and AstraZeneca for performing trials. The author and co‐authors of this manuscript have no financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
-
- Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention. NHLBI/WHO Workshop Report. Report No 02‐3659. Bethesda, MD: National Institutes of Health, National Heart Lung and Blood Institute, 2002
-
- Laitinen L A, Laitinen A, Haahtela T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a beta 2‐agonist, terbutaline, on airway inflammation in newly diagnosed asthma: a randomized, double‐blind, parallel‐group controlled trial. J Allergy Clin Immunol 19929032–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases