Computational model of in vivo human energy metabolism during semistarvation and refeeding
- PMID: 16449298
- PMCID: PMC2377067
- DOI: 10.1152/ajpendo.00523.2005
Computational model of in vivo human energy metabolism during semistarvation and refeeding
Abstract
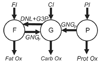
Changes in body weight and composition are the result of complex interactions among metabolic fluxes contributing to macronutrient balances. To better understand these interactions, a mathematical model was constructed that used the measured dietary macronutrient intake during semistarvation and refeeding as model inputs and computed whole body energy expenditure, de novo lipogenesis, and gluconeogenesis as well as turnover and oxidation of carbohydrate, fat, and protein. Published in vivo human data provided the basis for the model components that were integrated by fitting a few unknown parameters to the classic Minnesota human starvation experiment. The model simulated the measured body weight and fat mass changes during semistarvation and refeeding and predicted the unmeasured metabolic fluxes underlying the body composition changes. The resting metabolic rate matched the experimental measurements and required a model of adaptive thermogenesis. Refeeding caused an elevation of de novo lipogenesis that, along with increased fat intake, resulted in a rapid repletion and overshoot of body fat. By continuing the computer simulation with the prestarvation diet and physical activity, the original body weight and composition were eventually restored, but body fat mass was predicted to take more than one additional year to return to within 5% of its original value. The model was validated by simulating a recently published short-term caloric restriction experiment without changing the model parameters. The predicted changes in body weight, fat mass, resting metabolic rate, and nitrogen balance matched the experimental measurements, thereby providing support for the validity of the model.
Figures












References
-
- Aarsland A, Chinkes D, Wolfe RR. Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Am J Clin Nutr. 1997;65:1774–1782. - PubMed
-
- Acheson KJ, Schutz Y, Bessard T, Anantharaman K, Flatt JP, Jequier E. Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Am J Clin Nutr. 1988;48:240–247. - PubMed
-
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. 3rd ed. New York: Garland; 1994.
-
- Alpert SS. A two-reservoir energy model of the human body. Am J Clin Nutr. 1979;32:1710–1718. - PubMed
-
- Alpert SS. A limit on the energy transfer rate from the human fat store in hypophagia. J Theor Biol. 2005;233:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

