Presenilin-1 uses phospholipase D1 as a negative regulator of beta-amyloid formation
- PMID: 16449386
- PMCID: PMC1413665
- DOI: 10.1073/pnas.0510708103
Presenilin-1 uses phospholipase D1 as a negative regulator of beta-amyloid formation
Abstract
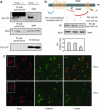
Presenilin (PS1/PS2) is a major component of gamma-secretase, the activity that mediates proteolysis of beta-amyloid precursor protein to generate beta-amyloid (Abeta). Here we demonstrate that PS1, through its loop region, binds to phospholipase D1 (PLD1), thereby recruiting it to the Golgi/trans-Golgi network. Overexpression of wild-type PLD1 reduces Abeta generation. Conversely, down-regulation of endogenous PLD1 by small hairpin RNA elevates Abeta production. The Abeta-lowering effect of PLD1 is independent of its ability to promote vesicular budding of beta-amyloid precursor protein. The data indicate that overexpression of PLD1 decreases, and down-regulation of PLD1 increases, the catalytic activity, and the association of the subunits, of gamma-secretase.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

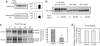


References
-
- Haass C., De Strooper B. Science. 1999;286:916–919. - PubMed
-
- De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. Nature. 1998;391:387–390. - PubMed
-
- Borchelt D. R., Thinakaran G., Eckman C. B., Lee M. K., Davenport F., Ratovitsky T., Prada C. M., Kim G., Seekins S., Yager D., et al. Neuron. 1996;17:1005–1013. - PubMed
-
- Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W., et al. Nat. Med. 1996;2:864–870. - PubMed
-
- Sisodia S. S., St George-Hyslop P. H. Nat. Rev. Neurosci. 2002;3:281–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

