Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes
- PMID: 16449571
- PMCID: PMC1369042
- DOI: 10.1101/gad.367606
Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes
Abstract
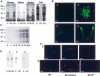
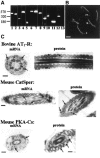
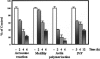
It is widely accepted that spermatozoa are translationally silent. The present study demonstrates, for the first time, incorporation of labeled amino acids into polypeptides during sperm capacitation, which was completely inhibited by mitochondrial translation inhibitors but not by the cytoplasmic translation inhibitor. Unlike 80S cytoplasmic ribosomes, 55S mitochondrial ribosomes were present in polysomal fractions, indicating that these ribosomes are actively involved in protein translation in spermatozoa. Inhibition of protein translation significantly reduced sperm motility, capacitation and in vitro fertilization rate. Thus, contrary to the accepted dogma, nuclear genes are expressed as proteins in sperm during their residence in the female reproductive tract until fertilization.
Figures

Similar articles
-
Role of translation by mitochondrial-type ribosomes during sperm capacitation: an analysis based on a proteomic approach.Proteomics. 2009 Mar;9(5):1385-99. doi: 10.1002/pmic.200800353. Proteomics. 2009. PMID: 19253287
-
Protein synthesis in sperm: dialog between mitochondria and cytoplasm.Mol Cell Endocrinol. 2008 Jan 30;282(1-2):45-55. doi: 10.1016/j.mce.2007.11.015. Epub 2007 Nov 22. Mol Cell Endocrinol. 2008. PMID: 18226442 Review.
-
De Novo Protein Synthesis Occurs Through the Cytoplasmic Translation Machinery in Mammalian Spermatozoa.J Cell Physiol. 2025 May;240(5):e70038. doi: 10.1002/jcp.70038. J Cell Physiol. 2025. PMID: 40373039
-
The presence and function of dopamine type 2 receptors in boar sperm: a possible role for dopamine in viability, capacitation, and modulation of sperm motility.Biol Reprod. 2009 Apr;80(4):753-61. doi: 10.1095/biolreprod.108.070961. Epub 2008 Dec 10. Biol Reprod. 2009. PMID: 19074002
-
Implications of diversity in sperm size and function for sperm competition and fertility.Int J Dev Biol. 2008;52(5-6):439-47. doi: 10.1387/ijdb.082595mg. Int J Dev Biol. 2008. PMID: 18649256 Review.
Cited by
-
Heat shock protein 90 has roles in intracellular calcium homeostasis, protein tyrosine phosphorylation regulation, and progesterone-responsive sperm function in human sperm.PLoS One. 2014 Dec 26;9(12):e115841. doi: 10.1371/journal.pone.0115841. eCollection 2014. PLoS One. 2014. PMID: 25541943 Free PMC article.
-
Cell-free seminal mRNA and microRNA exist in different forms.PLoS One. 2012;7(4):e34566. doi: 10.1371/journal.pone.0034566. Epub 2012 Apr 10. PLoS One. 2012. PMID: 22506029 Free PMC article.
-
In vitro impact of ethanolic extract of Bryonia laciniosa seed on Gir bull spermatozoa: a comprehensive evaluation through transcriptome profiling.Front Vet Sci. 2024 Jul 12;11:1419573. doi: 10.3389/fvets.2024.1419573. eCollection 2024. Front Vet Sci. 2024. PMID: 39071790 Free PMC article.
-
Differential Genes Expression between Fertile and Infertile Spermatozoa Revealed by Transcriptome Analysis.PLoS One. 2015 May 14;10(5):e0127007. doi: 10.1371/journal.pone.0127007. eCollection 2015. PLoS One. 2015. PMID: 25973848 Free PMC article.
-
Systematic variation in mRNA 3'-processing signals during mouse spermatogenesis.Nucleic Acids Res. 2007;35(1):234-46. doi: 10.1093/nar/gkl919. Epub 2006 Dec 8. Nucleic Acids Res. 2007. PMID: 17158511 Free PMC article.
References
-
- Alcivar A.A., Hake, L.E., Millette, C.F., Trasler, J.M., and Hecht, N.B. 1989. Mitochondrial gene expression in male germ cells of the mouse. Dev. Biol. 135: 263–271. - PubMed
-
- Bartoov B., Ben-Barak, J., Mayevsky, A., Sneider, M.Y.L., and Lightman, A. 1991. Sperm motility index: A new parameter for human sperm evaluation. Fertil. Steril. 56: 108–112. - PubMed
-
- Bragg P.W. and Handel, M.A. 1979. Protein synthesis in mouse spermatozoa. Biol. Reprod. 20: 333–337. - PubMed
-
- Breitbart H. 2003. Signaling pathways in sperm capacitation and acrosome reaction. Cell. Mol. Biol. 49: 321–327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
