Fox-2 mediates epithelial cell-specific fibroblast growth factor receptor 2 exon choice
- PMID: 16449636
- PMCID: PMC1367178
- DOI: 10.1128/MCB.26.4.1209-1222.2006
Fox-2 mediates epithelial cell-specific fibroblast growth factor receptor 2 exon choice
Abstract
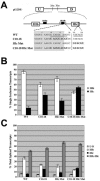
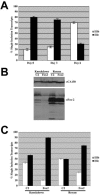
Alternative splicing of fibroblast growth factor receptor 2 (FGFR2) transcripts occurs in a cell-type-specific manner leading to the mutually exclusive use of exon IIIb in epithelia or exon IIIc in mesenchyme. Epithelial cell-specific exon choice is dependent on (U)GCAUG elements, which have been shown to bind Fox protein family members. In this paper we show that FGFR2 exon choice is regulated by (U)GCAUG elements and Fox protein family members. Fox-2 isoforms are differentially expressed in IIIb+ cells in comparison to IIIc+ cells, and expression of Fox-1 or Fox-2 in the latter led to a striking alteration in FGFR2 splice choice from IIIc to IIIb. This switch was absolutely dependent on the (U)GCAUG elements present in the FGFR2 pre-mRNA and required critical residues in the C-terminal region of Fox-2. Interestingly, Fox-2 expression led to skipping of exon 6 among endogenous Fox-2 transcripts and formation of an inactive Fox-2 isoform, which suggests that Fox-2 can regulate its own activity. Moreover, the repression of exon IIIc in IIIb+ cells was abrogated by interfering RNA-mediated knockdown of Fox-2. We also show that Fox-2 is critical for the FGFR2(IIIb)-to-FGFR2(IIIc) switch observed in T Rex-293 cells grown to overconfluency. Overconfluent T Rex-293 cells show molecular and morphological changes consistent with a mesenchymal-to-epithelial transition. If overconfluent cells are depleted of Fox-2, the switch from IIIc to IIIb is abrogated. The data in this paper place Fox-2 among critical regulators of gene expression during mesenchymal-epithelial transitions and demonstrate that this action of Fox-2 is mediated by mechanisms distinct from those described for other cases of Fox activity.
Figures





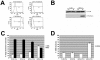


References
-
- Burns, R. C., T. J. Fairbanks, F. Sala, S. De Langhe, A. Mailleux, J. P. Thiery, C. Dickson, N. Itoh, D. Warburton, K. D. Anderson, and S. Bellusci. 2004. Requirement for fibroblast growth factor 10 or fibroblast growth factor receptor 2-IIIb signaling for cecal development in mouse. Dev. Biol. 265:61-74. - PubMed
-
- Carstens, R. P., J. V. Eaton, H. R. Krigman, P. J. Walther, and M. A. Garcia-Blanco. 1997. Alternative splicing of fibroblast growth factor receptor 2 (FGF-R2) in human prostate cancer. Oncogene 15:3059-3065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
