Evolution and development of Hertwig's epithelial root sheath
- PMID: 16450392
- PMCID: PMC2734338
- DOI: 10.1002/dvdy.20674
Evolution and development of Hertwig's epithelial root sheath
Abstract
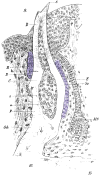
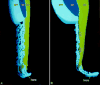
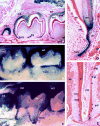
Periodontal regeneration and tissue engineering has re-awakened interest in the role of Hertwig's Epithelial Root Sheath (HERS), an epithelial tissue layer first discovered in amphibians more than a century ago. Using developmental, evolutionary, and cell biological approaches, we have, therefore, performed a careful analysis of the role of HERS in root formation and compared our data with clinical findings. Our developmental studies revealed HERS as a transient structure assembled in the early period of root formation and elongation and, subsequently, fenestrated and reduced to epithelial rests of Malassez (ERM). Our comparative evolutionary studies indicated that HERS fenestration was closely associated with the presence of a periodontal ligament and a gomphosis-type attachment apparatus in crocodilians and mammals. Based on these studies, we are proposing that HERS plays an important role in the regulation and maintenance of periodontal ligament space and function. Additional support for this hypothesis was rendered by our meta-analysis of recent clinical reports related to HERS function.
(c) 2006 Wiley-Liss, Inc.
Figures









References
-
- Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. - PubMed
-
- Alatli I, Lundmark C, Hammarstrom L. The localization of epithelial root sheath cells during cementum formation in rat molars. J Periodontal Res. 1996;31:433–440. - PubMed
-
- Alatli I, Lundmark C, Hammarstrom L. The localization of epithelial root sheath cells during cementum formation in rat molars. J Periodontal Res. 1996;31:433–440. - PubMed
-
- Andreasen JO, Paulsen HU, Yu Z, Bayer T. A long-term study of 370 autotransplanted premolars. Part IV. Root development subsequent to transplantation. Eur J Orthod. 1990;12:38–50. - PubMed
-
- Andujar MB, Magloire H, Grimaud JA. Fibronectin in basement membrane of Hertwig’s epithelial sheath. Light and electron immunohistochemical localization. Histochemistry. 1984;81:279–282. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

