Transgenic isolation of skeletal muscle and kidney defects in laminin beta2 mutant mice: implications for Pierson syndrome
- PMID: 16452099
- PMCID: PMC1363729
- DOI: 10.1242/dev.02270
Transgenic isolation of skeletal muscle and kidney defects in laminin beta2 mutant mice: implications for Pierson syndrome
Abstract
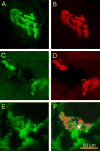
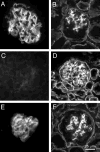
Pierson syndrome is a recently defined disease usually lethal within the first postnatal months and caused by mutations in the gene encoding laminin beta2 (LAMB2). The hallmarks of Pierson syndrome are congenital nephrotic syndrome accompanied by ocular abnormalities, including microcoria (small pupils), with muscular and neurological developmental defects also present. Lamb2(-/-) mice are a model for Pierson syndrome; they exhibit defects in the kidney glomerular barrier, in the development and organization of the neuromuscular junction, and in the retina. Lamb2(-/-) mice fail to thrive and die very small at 3 weeks of age, but to what extent the kidney and neuromuscular defects each contribute to this severe phenotype has been obscure, though highly relevant to understanding Pierson syndrome. To investigate this, we generated transgenic mouse lines expressing rat laminin beta2 either in muscle or in glomerular epithelial cells (podocytes) and crossed them onto the Lamb2(-/-) background. Rat beta2 was confined in skeletal muscle to synapses and myotendinous junctions, and in kidney to the glomerular basement membrane. In transgenic Lamb2(-/-) mice, beta2 deposition in only glomeruli prevented proteinuria but did not ameliorate the severe phenotype. By contrast, beta2 expression in only muscle restored synaptic architecture and led to greatly improved health, but the mice died from kidney disease at 1 month. Rescue of both glomeruli and synapses was associated with normal weight gain, fertility and lifespan. We conclude that muscle defects in Lamb2(-/-) mice are responsible for the severe failure to thrive phenotype, and that renal replacement therapy alone will be an inadequate treatment for Pierson syndrome.
Figures






References
-
- Abrahamson DR, Irwin MH, St John PL, Perry EW, Accavitti MA, Heck LW, Couchman JR. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol. 1989;109:3477–3491. - PMC - PubMed
-
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JCR, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. - PubMed
-
- Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997;9:651–658. - PubMed
-
- Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE. Glomerular-Specific Gene Excision In Vivo. J Am Soc Nephrol. 2002;13:788–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

