Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland
- PMID: 16452162
- PMCID: PMC1413744
- DOI: 10.1073/pnas.0510974103
Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland
Abstract
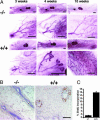
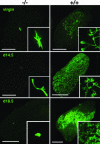
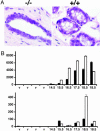
Estradiol is a major regulator of postnatal mammary gland development and thought to exert its effects through estrogen receptor alpha (ERalpha) expressed in the mammary gland stroma and epithelium. Previous studies, however, were confounded by the use of an ERalpha mutant strain that retains some of the protein with transactivation activity. Here, we use an ERalpha-/- mouse strain in which no ERalpha transcript can be detected to analyze mammary gland development in the complete absence of ERalpha signaling. The ERalpha-/- females show no development beyond a rudimentary ductal system. By grafting ERalpha-/- epithelium or stroma in combination with ERalpha WT stroma or epithelium, we show that the primary target for estradiol is the mammary epithelium, whereas a direct response of the mammary stroma is not required for mammary gland development to proceed normally. Mammary glands reconstituted with ERalpha-/- mammary epithelium exposed to pregnancy hormones show increased transcription of milk protein genes, indicating that ERalpha signaling is not an absolute requirement for a transcriptional response to pregnancy hormones. When ERalpha-/- mammary epithelial cells are in close vicinity to ERalpha WT cells, they proliferate and contribute to all aspects of mammary gland development, indicating that estradiol, like progesterone, orchestrates proliferation and morphogenesis by a paracrine mechanism, affecting nearby cells in the mammary epithelium.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
-
- Daniel C. W., Silberstein G. B. In: The Mammary Gland. Daniel C. W., editor. New York: Plenum; 1987. pp. 3–31.
-
- Brisken C. J. Mammary Gland Biol. Neoplasia. 2002;7:39–48. - PubMed
-
- Nandi S. J. Natl. Cancer Inst. 1958;21:1039–1063. - PubMed
-
- Lyons W. R. Proc. R. Soc. London Ser. B; 1958. pp. 303–325. - PubMed
-
- Lydon J. P., DeMayo F. J., Funk C. R., Mani S. K., Hughes A. R., Montgomery C. A., Jr, Shyamala G., Conneely O. M., O’Malley B. W. Genes Dev. 1995;9:2266–2278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

