Crystal structure of human apolipoprotein A-I: insights into its protective effect against cardiovascular diseases
- PMID: 16452169
- PMCID: PMC1413691
- DOI: 10.1073/pnas.0506877103
Crystal structure of human apolipoprotein A-I: insights into its protective effect against cardiovascular diseases
Retraction in
-
Retraction for Ajees et al., Crystal structure of human apolipoprotein A-I: Insights into its protective effect against cardiovascular diseases.Proc Natl Acad Sci U S A. 2018 Jul 17;115(29):E6966. doi: 10.1073/pnas.1806430115. Epub 2018 Jul 9. Proc Natl Acad Sci U S A. 2018. PMID: 29987041 Free PMC article. No abstract available.
Expression of concern in
-
Editorial Expression of Concern for multiple articles.Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6551. doi: 10.1073/pnas.1003210107. Proc Natl Acad Sci U S A. 2010. PMID: 28277629 Free PMC article. No abstract available.
Abstract
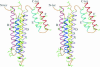
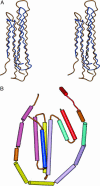
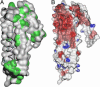
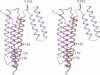
Despite three decades of extensive studies on human apolipoprotein A-I (apoA-I), the major protein component in high-density lipoproteins, the molecular basis for its antiatherogenic function is elusive, in part because of lack of a structure of the full-length protein. We describe here the crystal structure of lipid-free apoA-I at 2.4 A. The structure shows that apoA-I is comprised of an N-terminal four-helix bundle and two C-terminal helices. The N-terminal domain plays a prominent role in maintaining its lipid-free conformation, indicating that mutants with truncations in this region form inadequate models for explaining functional properties of apoA-I. A model for transformation of the lipid-free conformation to the high-density lipoprotein-bound form follows from an analysis of solvent-accessible hydrophobic patches on the surface of the structure and their proximity to the hydrophobic core of the four-helix bundle. The crystal structure of human apoA-I displays a hitherto-unobserved array of positively and negatively charged areas on the surface. Positioning of the charged surface patches relative to hydrophobic regions near the C terminus of the protein offers insights into its interaction with cell-surface components of the reverse cholesterol transport pathway and antiatherogenic properties of this protein. This structure provides a much-needed structural template for exploration of molecular mechanisms by which human apoA-I ameliorates atherosclerosis and inflammatory diseases.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Comment in
-
Findings of Research Misconduct.Fed Regist. 2018 Apr 16;83(73):16370-16371. Fed Regist. 2018. PMID: 30407470 Free PMC article. No abstract available.
References
-
- Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. J. Am. Med. Assoc. 2003;290:2292–2300. - PubMed
-
- Glomset J. A. J. Lipid Res. 1968;9:155–167. - PubMed
-
- Fielding P. E., Nagao K., Hakamata H., Chimini G., Fielding C. J. Biochemistry. 2000;39:14113–14120. - PubMed
-
- Brewer H. B., Jr Arterioscler. Thromb. Vasc. Biol. 2004;24:387–391. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

