mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt
- PMID: 16452206
- PMCID: PMC3193604
- DOI: 10.1158/0008-5472.CAN-05-2925
mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt
Abstract
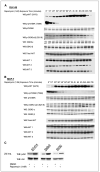
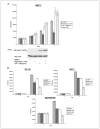
Stimulation of the insulin and insulin-like growth factor I (IGF-I) receptor activates the phosphoinositide-3-kinase/Akt/mTOR pathway causing pleiotropic cellular effects including an mTOR-dependent loss in insulin receptor substrate-1 expression leading to feedback down-regulation of signaling through the pathway. In model systems, tumors exhibiting mutational activation of phosphoinositide-3-kinase/Akt kinase, a common event in cancers, are hypersensitive to mTOR inhibitors, including rapamycin. Despite the activity in model systems, in patients, mTOR inhibitors exhibit more modest antitumor activity. We now show that mTOR inhibition induces insulin receptor substrate-1 expression and abrogates feedback inhibition of the pathway, resulting in Akt activation both in cancer cell lines and in patient tumors treated with the rapamycin derivative, RAD001. IGF-I receptor inhibition prevents rapamycin-induced Akt activation and sensitizes tumor cells to inhibition of mTOR. In contrast, IGF-I reverses the antiproliferative effects of rapamycin in serum-free medium. The data suggest that feedback down-regulation of receptor tyrosine kinase signaling is a frequent event in tumor cells with constitutive mTOR activation. Reversal of this feedback loop by rapamycin may attenuate its therapeutic effects, whereas combination therapy that ablates mTOR function and prevents Akt activation may have improved antitumor activity.
Figures




Comment in
-
How Compensatory Mechanisms and Adaptive Rewiring Have Shaped Our Understanding of Therapeutic Resistance in Cancer.Cancer Res. 2021 Dec 15;81(24):6074-6077. doi: 10.1158/0008-5472.CAN-21-3605. Cancer Res. 2021. PMID: 34911779 Free PMC article.
References
-
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. - PubMed
-
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. - PubMed
-
- Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 2003;13:79–85. - PubMed
-
- Haruta T, Uno T, Kawahara J, et al. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–94. - PubMed
-
- Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001;50:24–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

