Archaeal and bacterial SecD and SecF homologs exhibit striking structural and functional conservation
- PMID: 16452406
- PMCID: PMC1367261
- DOI: 10.1128/JB.188.4.1251-1259.2006
Archaeal and bacterial SecD and SecF homologs exhibit striking structural and functional conservation
Abstract
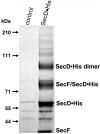
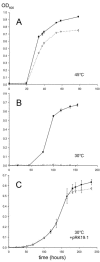
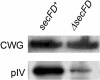
The majority of secretory proteins are translocated into and across hydrophobic membranes via the universally conserved Sec pore. Accessory proteins, including the SecDF-YajC Escherichia coli membrane complex, are required for efficient protein secretion. E. coli SecDF-YajC has been proposed to be involved in the membrane cycling of SecA, the cytoplasmic bacterial translocation ATPase, and in the stabilizing of SecG, a subunit of the Sec pore. While there are no identified archaeal homologs of either SecA or SecG, many archaea possess homologs of SecD and SecF. Here, we present the first study that addresses the function of archaeal SecD and SecF homologs. We show that the SecD and SecF components in the model archaeon Haloferax volcanii form a cytoplasmic membrane complex in the native host. Furthermore, as in E. coli, an H. volcanii deltasecFD mutant strain exhibits both severe cold sensitivity and a Sec-specific protein translocation defect. Taken together, these results demonstrate significant functional conservation among the prokaryotic SecD and SecF homologs despite the distinct composition of their translocation machineries.
Figures
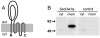


References
-
- Cao, T. B., and M. H. Saier, Jr. 2003. The general protein secretory pathway: phylogenetic analyses leading to evolutionary conclusions. Biochim. Biophys. Acta 1609:115-125. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

