lbtA and lbtB are required for production of the Legionella pneumophila siderophore legiobactin
- PMID: 16452417
- PMCID: PMC1367248
- DOI: 10.1128/JB.188.4.1351-1363.2006
lbtA and lbtB are required for production of the Legionella pneumophila siderophore legiobactin
Abstract
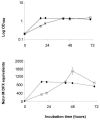
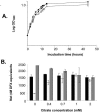
Under iron stress, Legionella pneumophila secretes legiobactin, a nonclassical siderophore that is reactive in the chrome azurol S (CAS) assay. Here, we have optimized conditions for legiobactin expression, shown its biological activity, and identified two genes, lbtA and lbtB, which are involved in legiobactin production. lbtA appears to be iron repressed and encodes a protein that has significant homology with siderophore synthetases, and FrgA, a previously described iron-regulated protein of L. pneumophila. lbtB encodes a protein homologous with members of the major facilitator superfamily of multidrug efflux pumps. Mutants lacking lbtA or lbtB were defective for legiobactin, producing 40 to 70% less CAS reactivity in deferrated chemically defined medium (CDM). In bioassays, mutant CDM culture supernatants, unlike those of the wild type, did not support growth of iron-limited wild-type bacteria in 2',2'-dipyridyl-containing buffered charcoal yeast extract (BCYE) agar and a ferrous iron transport mutant on BCYE agar without added iron. The lbtA mutant was modestly defective for growth in deferrated CDM containing the iron chelator citrate, indicating that legiobactin is required in conditions of severe iron limitation. Complementation of the lbt mutants restored both siderophore expression, as measured by the CAS assay and bioassays, and bacterial growth in deferrated, citrate-containing media. The lbtA mutant replicated as the wild type did in macrophages, amoebae, and the lungs of mice. However, L. pneumophila expresses lbtA in the macrophage, suggesting that legiobactin, though not required, may play a dispensable role in intracellular growth. The discovery of lbtAB represents the first identification of genes required for L. pneumophila siderophore expression.
Figures





References
-
- Abu Kwaik, Y. 1998. Fatal attraction of mammalian cells to Legionella pneumophila. Mol. Microbiol. 30:689-695. - PubMed
-
- Arnow, L. E. 1937. Colorimetric determination of the components of 3,4-dihyrdoxyphenylalanine tyrosine mixtures. J. Biol. Chem. 118:531-537.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

