Quorum sensing in Yersinia enterocolitica controls swimming and swarming motility
- PMID: 16452428
- PMCID: PMC1367215
- DOI: 10.1128/JB.188.4.1451-1461.2006
Quorum sensing in Yersinia enterocolitica controls swimming and swarming motility
Abstract
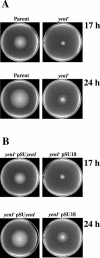
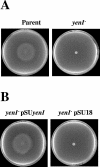
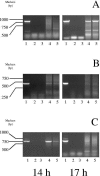
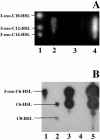
The Yersinia enterocolitica LuxI homologue YenI directs the synthesis of N-3-(oxohexanoyl)homoserine lactone (3-oxo-C6-HSL) and N-hexanoylhomoserine lactone (C6-HSL). In a Y. enterocolitica yenI mutant, swimming motility is temporally delayed while swarming motility is abolished. Since both swimming and swarming are flagellum dependent, we purified the flagellin protein from the parent and yenI mutant. Electrophoresis revealed that in contrast to the parent strain, the yenI mutant grown for 17 h at 26 degrees C lacked the 45-kDa flagellin protein FleB. Reverse transcription-PCR indicated that while mutation of yenI had no effect on yenR, flhDC (the motility master regulator) or fliA (the flagellar sigma factor) expression, fleB (the flagellin structural gene) was down-regulated. Since 3-oxo-C6-HSL and C6-HSL did not restore swimming or swarming in the yenI mutant, we reexamined the N-acylhomoserine lactone (AHL) profile of Y. enterocolitica. Using AHL biosensors and mass spectrometry, we identified three additional AHLs synthesized via YenI: N-(3-oxodecanoyl)homoserine lactone, N-(3-oxododecanoyl)homoserine lactone (3-oxo-C12-HSL), and N-(3-oxotetradecanoyl)homoserine lactone. However, none of the long-chain AHLs either alone or in combination with the short-chain AHLs restored swarming or swimming in the yenI mutant. By investigating the transport of radiolabeled 3-oxo-C12-HSL and by introducing an AHL biosensor into the yenI mutant we demonstrate that the inability of exogenous AHLs to restore motility to the yenI mutant is not related to a lack of AHL uptake. However, both AHL synthesis and motility were restored by complementation of the yenI mutant with a plasmid-borne copy of yenI.
Figures



References
-
- Allison, C., L. Emody, N. Coleman, and C. Hughes. 1994. The role of swarm cell-differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J. Infect. Dis. 169:1155-1158. - PubMed
-
- Atkinson, S., R. E. Sockett, M. Camara, and Williams, P. 2004. N-Acylhomoserine lactone mediated quorum sensing in Yersinia, p. 75-90. In E. Carniel and B. J. Hinnebusch (ed.), Yersinia: molecular and cellular biology. Horizon Bioscience, Wymondham, United Kingdom.
-
- Atkinson, S., J. P. Throup, G. S. A. B. Stewart, and P. Williams. 1999. A hierarchical quorum sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol. Microbiol. 33:1267-1277. - PubMed
-
- Bartolome, B., Y. Jubete, E. Martinez, and F. Delacruz. 1991. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102:75-78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

