Sex-lethal imparts a sex-specific function to UNR by recruiting it to the msl-2 mRNA 3' UTR: translational repression for dosage compensation
- PMID: 16452508
- PMCID: PMC1361707
- DOI: 10.1101/gad.371406
Sex-lethal imparts a sex-specific function to UNR by recruiting it to the msl-2 mRNA 3' UTR: translational repression for dosage compensation
Abstract
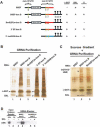
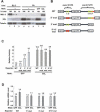
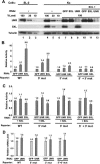
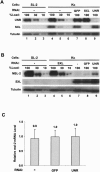
MSL-2 (male-specific lethal 2) is the limiting component of the Drosophila dosage compensation complex (DCC) that specifically increases transcription from the male X chromosome. Ectopic expression of MSL-2 protein in females causes DCC assembly on both X chromosomes and lethality. Inhibition of MSL-2 synthesis requires the female-specific protein sex-lethal (SXL), which binds to the msl-2 mRNA 5' and 3' untranslated regions (UTRs) and blocks translation through distinct UTR-specific mechanisms. Here, we purify translationally silenced msl-2 mRNPs and identify UNR (upstream of N-ras) as a protein recruited to the 3' UTR by SXL. We demonstrate that SXL requires UNR as a corepressor for 3'-UTR-mediated regulation, imparting a female-specific function to the ubiquitously expressed UNR protein. Our results reveal a novel functional role for UNR as a translational repressor and indicate that UNR is a key component of a "fail-safe" dosage compensation regulatory system that prevents toxic MSL-2 synthesis in female cells.
Figures


Similar articles
-
Drosophila UNR is required for translational repression of male-specific lethal 2 mRNA during regulation of X-chromosome dosage compensation.Genes Dev. 2006 Feb 1;20(3):380-9. doi: 10.1101/gad.371906. Genes Dev. 2006. PMID: 16452509 Free PMC article.
-
The SXL-UNR corepressor complex uses a PABP-mediated mechanism to inhibit ribosome recruitment to msl-2 mRNA.Mol Cell. 2009 Nov 25;36(4):571-82. doi: 10.1016/j.molcel.2009.09.042. Mol Cell. 2009. PMID: 19941818
-
Functional domains of Drosophila UNR in translational control.RNA. 2008 Mar;14(3):482-90. doi: 10.1261/rna.802908. Epub 2008 Jan 18. RNA. 2008. PMID: 18203923 Free PMC article.
-
Regulation of the expression and activity of Unr in mammalian cells.Biochem Soc Trans. 2015 Dec;43(6):1241-6. doi: 10.1042/BST20150165. Biochem Soc Trans. 2015. PMID: 26614667 Review.
-
Path to equality strewn with roX.Dev Biol. 2004 May 1;269(1):18-25. doi: 10.1016/j.ydbio.2004.01.039. Dev Biol. 2004. PMID: 15081354 Review.
Cited by
-
Translation initiation by the c-myc mRNA internal ribosome entry sequence and the poly(A) tail.RNA. 2008 Aug;14(8):1579-89. doi: 10.1261/rna.1043908. Epub 2008 Jun 12. RNA. 2008. PMID: 18556416 Free PMC article.
-
Sequence-specific targeting of dosage compensation in Drosophila favors an active chromatin context.PLoS Genet. 2012;8(4):e1002646. doi: 10.1371/journal.pgen.1002646. Epub 2012 Apr 26. PLoS Genet. 2012. PMID: 22570616 Free PMC article.
-
Hrp48 and eIF3d contribute to msl-2 mRNA translational repression.Nucleic Acids Res. 2018 May 4;46(8):4099-4113. doi: 10.1093/nar/gky246. Nucleic Acids Res. 2018. PMID: 29635389 Free PMC article.
-
Dosage compensation in Drosophila melanogaster: epigenetic fine-tuning of chromosome-wide transcription.Nat Rev Genet. 2012 Jan 18;13(2):123-34. doi: 10.1038/nrg3124. Nat Rev Genet. 2012. PMID: 22251873 Review.
-
Sex-lethal promotes nuclear retention of msl2 mRNA via interactions with the STAR protein HOW.Genes Dev. 2013 Jun 15;27(12):1421-33. doi: 10.1101/gad.214999.113. Genes Dev. 2013. PMID: 23788626 Free PMC article.
References
-
- Arbeitman M.N., Furlong, E.E., Imam, F., Johnson, E., Null, B.H., Baker, B.S., Krasnow, M.A., Scott, M.P., Davis, R.W., and White, K.P. 2002. Gene expression during the life cycle of Drosophila melanogaster. Science 297: 2270-2275. - PubMed
-
- Ausubel F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. 1999. Short protocols in molecular biology. John Wiley & Sons, Inc., New York.
-
- Baker B.S., Gorman, M., and Marin, I. 1994. Dosage compensation in Drosophila. Annu. Rev. Genet. 28: 491-521. - PubMed
-
- Bashaw G.J. and Baker, B.S. 1995. The msl-2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal. Development 121: 3245-3258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
