Drosophila UNR is required for translational repression of male-specific lethal 2 mRNA during regulation of X-chromosome dosage compensation
- PMID: 16452509
- PMCID: PMC1361708
- DOI: 10.1101/gad.371906
Drosophila UNR is required for translational repression of male-specific lethal 2 mRNA during regulation of X-chromosome dosage compensation
Abstract
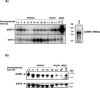
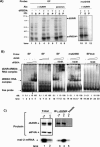
The inhibition of male-specific lethal 2 (msl-2) mRNA translation by the RNA-binding protein sex-lethal (SXL) is an essential regulatory step for X-chromosome dosage compensation in Drosophila melanogaster. The mammalian upstream of N-ras (UNR) protein has been implicated in the regulation of mRNA stability and internal ribosome entry site (IRES)-dependent mRNA translation. Here we have identified the Drosophila homolog of mammalian UNR as a cofactor required for SXL-mediated repression of msl-2 translation. UNR interacts with SXL, a female-specific protein. Although UNR is present in both male and female flies, binding of SXL to uridine-rich sequences in the 3' untranslated region (UTR) of msl-2 mRNA recruits UNR to adjacent regulatory sequences, thereby conferring a sex-specific function to UNR. These data identify a novel regulator of dosage compensation in Drosophila that acts coordinately with SXL in translational control.
Figures



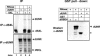
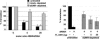
References
-
- Arbeitman M.N., Furlong, E.E., Imam, F., Johnson, E., Null, B.H., Baker, B.S., Krasnow, M.A., Scott, M.P., Davis, R.W., and White, K.P. 2002. Science 297: 2270-2275. - PubMed
-
- Bashaw G.J. and Baker, B.S. 1997. The regulation of the Drosophila msl-2 gene reveals a function for Sex-lethal in translational control. Cell 89: 789-798. - PubMed
-
- Beckmann K., Grskovic, M., Gebauer, F., and Hentze, M.W. 2005. A dual inhibitory mechanism for ribosomal 43S complex recruitment and scanning restricts msl-2 mRNA translation for dosage compensation in Drosophila. Cell 122: 529-540. - PubMed
-
- Bopp D., Bell, L.R., Cline, T.W., and Schedl, P. 1991. Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes & Dev. 5: 403-415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
