Fluorescence resonance energy transfer and molecular modeling studies on 4',6-diamidino-2-phenylindole (DAPI) complexes with tubulin
- PMID: 16452620
- PMCID: PMC2249762
- DOI: 10.1110/ps.051862206
Fluorescence resonance energy transfer and molecular modeling studies on 4',6-diamidino-2-phenylindole (DAPI) complexes with tubulin
Abstract
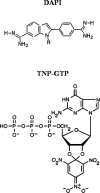
The goal of this work was to determine the binding properties and location of 4',6-diamidino-2-phenylindole (DAPI) complexed with tubulin. Using fluorescence anisotropy, a dissociation constant of 5.2+/-0.4 microM for the DAPI-tubulin complex was determined, slightly lower than that for the tubulin S complex. The influence of the C-terminal region on the binding of DAPI to tubulin was also characterized. Using FRET experiments, and assuming a kappa2 value of 2/3, distances between Co2+ bound to its high-affinity binding site and the DAPI-binding site and 2',3'-O-(trinitrophenyl)guanosine 5'-triphosphate bound to the exchangeable nucleotide and the DAPI-binding site were found to be 20+/-2 A and 43+/-2 A, respectively. To locate potential DAPI-binding sites on tubulin, a molecular modeling study was carried out using the tubulin crystal structure and energy minimization calculations. The results from the FRET measurements were used to limit the possible location of DAPI in the tubulin structure. Several candidate binding sites were found and these are discussed in the context of the various properties of bound DAPI.
Figures




Similar articles
-
Interaction between the C-terminal peptides of tubulin and tubulin S detected with the fluorescent probe 4',6-diamidino-2-phenylindole.Arch Biochem Biophys. 1993 May 15;303(1):159-64. doi: 10.1006/abbi.1993.1267. Arch Biochem Biophys. 1993. PMID: 8489260
-
Spectroscopic studies on ligand-enzyme interactions: complexation of alpha-chymotrypsin with 4',6-diamidino-2-phenylindole (DAPI).J Phys Chem B. 2008 Feb 14;112(6):1828-33. doi: 10.1021/jp709621c. Epub 2008 Jan 19. J Phys Chem B. 2008. PMID: 18205349
-
Distances between the paclitaxel, colchicine, and exchangeable GTP binding sites on tubulin.Biochemistry. 1998 May 12;37(19):6636-44. doi: 10.1021/bi9719760. Biochemistry. 1998. PMID: 9578547
-
DAPI: a DNA-specific fluorescent probe.Biotech Histochem. 1995 Sep;70(5):220-33. doi: 10.3109/10520299509108199. Biotech Histochem. 1995. PMID: 8580206 Review.
-
Fluorescent and colored trinitrophenylated analogs of ATP and GTP.Eur J Biochem. 2003 Sep;270(17):3479-85. doi: 10.1046/j.1432-1033.2003.03748.x. Eur J Biochem. 2003. PMID: 12919312 Review.
Cited by
-
4',6-Diamidino-2-Phenylindole Distinctly Labels Tau Deposits.J Histochem Cytochem. 2018 Oct;66(10):737-751. doi: 10.1369/0022155418793600. Epub 2018 Aug 14. J Histochem Cytochem. 2018. PMID: 30106598 Free PMC article.
-
Discovery of anti-TB agents that target the cell-division protein FtsZ.Future Med Chem. 2010 Aug;2(8):1305-23. doi: 10.4155/fmc.10.220. Future Med Chem. 2010. PMID: 21339840 Free PMC article. Review.
References
-
- Barcellona, M.L. and Gratton, E. 1990. The fluorescence properties of a DNA probe: 4′-6-Diamidino-2-phenylindole (DAPI). Eur. Biophys. J. 17: 315–323. - PubMed
-
- Bhattacharyya, B., Sackett, D. L., and Wolff, J. 1985. Tubulin hybrid dimers, and tubulin S. J. Biol. Chem. 260: 10208–10216. - PubMed
-
- Bhattacharyya, A., Bhattacharyya, B., and Roy, S. 1993. A study of colchicine tubulin complex by donor quenching of fluorescence energy transfer. Eur. J. Biochem. 216: 757–761. - PubMed
-
- Bhattacharyya, A. Bhattacharyya, B., and Roy, S. 1994. Magnesium-induced structural changes in tubulin. J. Biol. Chem. 269: 28655–28661. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

