Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin
- PMID: 16452635
- PMCID: PMC1415305
- DOI: 10.1091/mbc.e05-07-0607
Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin
Abstract
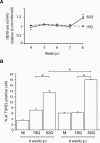
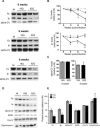
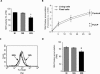
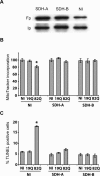
Alterations of mitochondrial function may play a central role in neuronal death in Huntington's disease (HD). However, the molecular mechanisms underlying such functional deficits of mitochondria are not elucidated yet. We herein showed that the expression of two important constituents of mitochondrial complex II, the 30-kDa iron-sulfur (Ip) subunit and the 70-kDa FAD (Fp) subunit, was preferentially decreased in the striatum of HD patients compared with controls. We also examined several mitochondrial proteins in striatal neurons that were infected with lentiviral vectors coding for the N-terminus part of huntingtin (Htt) with either a pathological (Htt171-82Q) or physiological (Htt171-19Q) polyglutamine tract. Compared with Htt171-19Q, expression of Htt171-82Q preferentially decreased the levels of Ip and Fp subunits and affected the dehydrogenase activity of the complex. The Htt171-82Q-induced preferential loss of complex II was not associated with a decrease in mRNA levels, suggesting the involvement of a posttranscriptional mechanism. Importantly, the overexpression of either Ip or Fp subunit restored complex II levels and blocked mitochondrial dysfunction and striatal cell death induced by Htt171-82Q in striatal neurons. The present results strongly suggest that complex II defects in HD may be instrumental in striatal cell death.
Figures


References
-
- Ackrell, B. A. (2000). Progress in understanding structure-function relationships in respiratory chain complex II. FEBS Lett. 466, 1–5. - PubMed
-
- Bae, B. I. et al. (2005). p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron 47, 29–41. - PubMed
-
- Beal, M. F. (1992). Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann. Neurol. 31, 119–130. - PubMed
-
- Benchoua, A., Braudeau, J., Reis, A., Couriaud, C., and Onteniente, B. (2004). Activation of proinflammatory caspases by cathepsin B in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 24, 1272–1279. - PubMed
-
- Benchoua, A., Couriaud, C., Guegan, C., Tartier, L., Couvert, P., Friocourt, G., Chelly, J., Menissier-de Murcia, J., and Onteniente, B. (2002). Active caspase-8 translocates into the nucleus of apoptotic cells to inactivate poly(ADP-ribose) polymerase-2. J. Biol. Chem. 277, 34217–34222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

