Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis
- PMID: 16452657
- PMCID: PMC6675492
- DOI: 10.1523/JNEUROSCI.2676-05.2006
Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis
Abstract
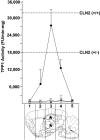
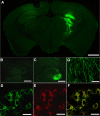
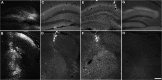
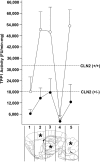
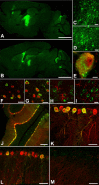
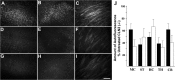
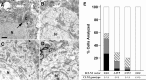
Classical late infantile neuronal ceroid lipofuscinosis (cLINCL) is a lysosomal storage disorder caused by mutations in CLN2, which encodes lysosomal tripeptidyl peptidase I (TPP1). Lack of TPP1 results in accumulation of autofluorescent storage material and curvilinear bodies in cells throughout the CNS, leading to progressive neurodegeneration and death typically in childhood. In this study, we injected adeno-associated virus (AAV) vectors containing the human CLN2 cDNA into the brains of CLN2(-/-) mice to determine therapeutic efficacy. AAV2CUhCLN2 or AAV5CUhCLN2 were stereotaxically injected into the motor cortex, thalamus, and cerebellum of both hemispheres at 6 weeks of age, and mice were then killed at 13 weeks after injection. Mice treated with AAV2CUhCLN2 and AAV5CUhCLN2 contained TPP1 activity at each injection tract that was equivalent to 0.5- and 2-fold that of CLN2(+/+) control mice, respectively. Lysosome-associated membrane protein 1 immunostaining and confocal microscopy showed intracellular targeting of TPP1 to the lysosomal compartment. Compared with control animals, there was a marked reduction of autofluorescent storage in the AAV2CUhCLN2 and AAV5CUhCLN2 injected brain regions, as well as adjacent regions, including the striatum and hippocampus. Analysis by electron microscopy confirmed a significant decrease in pathological curvilinear bodies in cells. This study demonstrates that AAV-mediated TPP1 enzyme replacement corrects the hallmark cellular pathologies of cLINCL in the mouse model and raises the possibility of using AAV gene therapy to treat cLINCL patients.
Figures
Similar articles
-
Timing of therapeutic intervention determines functional and survival outcomes in a mouse model of late infantile batten disease.Mol Ther. 2007 Oct;15(10):1782-8. doi: 10.1038/sj.mt.6300249. Epub 2007 Jul 17. Mol Ther. 2007. PMID: 17637720
-
Clinical protocol. Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis.Hum Gene Ther. 2004 Nov;15(11):1131-54. doi: 10.1089/hum.2004.15.1131. Hum Gene Ther. 2004. PMID: 15610613 Clinical Trial.
-
AAV2-mediated CLN2 gene transfer to rodent and non-human primate brain results in long-term TPP-I expression compatible with therapy for LINCL.Gene Ther. 2005 Nov;12(22):1618-32. doi: 10.1038/sj.gt.3302549. Gene Ther. 2005. PMID: 16052206
-
CLN2 Disease (Classic Late Infantile Neuronal Ceroid Lipofuscinosis).Pediatr Endocrinol Rev. 2016 Jun;13 Suppl 1:682-8. Pediatr Endocrinol Rev. 2016. PMID: 27491216 Review.
-
Feasibility of gene therapy for late neuronal ceroid lipofuscinosis.Arch Neurol. 2001 Nov;58(11):1793-8. doi: 10.1001/archneur.58.11.1793. Arch Neurol. 2001. PMID: 11708986 Review.
Cited by
-
Dipeptidyl-peptidase I does not functionally compensate for the loss of tripeptidyl-peptidase I in the neurodegenerative disease late-infantile neuronal ceroid lipofuscinosis.Biochem J. 2008 Oct 15;415(2):225-32. doi: 10.1042/BJ20080411. Biochem J. 2008. PMID: 18570628 Free PMC article.
-
Global Brain Transcriptome Analysis of a Tpp1 Neuronal Ceroid Lipofuscinoses Mouse Model.ASN Neuro. 2019 Jan-Dec;11:1759091419843393. doi: 10.1177/1759091419843393. ASN Neuro. 2019. PMID: 31003587 Free PMC article.
-
Clinical applications involving CNS gene transfer.Adv Genet. 2014;87:71-124. doi: 10.1016/B978-0-12-800149-3.00002-0. Adv Genet. 2014. PMID: 25311921 Free PMC article. Review.
-
Neuronal Ceroid Lipofuscinosis: Potential for Targeted Therapy.Drugs. 2021 Jan;81(1):101-123. doi: 10.1007/s40265-020-01440-7. Drugs. 2021. PMID: 33242182 Review.
-
Pathology associated with AAV mediated expression of beta amyloid or C100 in adult mouse hippocampus and cerebellum.PLoS One. 2013;8(3):e59166. doi: 10.1371/journal.pone.0059166. Epub 2013 Mar 13. PLoS One. 2013. PMID: 23516609 Free PMC article.
References
-
- Alisky JM, Hughes SM, Sauter SL, Jolly D, Dubensky TW, Staber PD, Chiorini JA, Davidson BL (2000). Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. NeuroReport 11:2669–2673. - PubMed
-
- Autti T, Raininko R, Vanhanen SL, Santavuori P (1997). Magnetic resonance techniques in neuronal ceroid lipofuscinoses and some other lysosomal diseases affecting the brain. Curr Opin Neurol 10:519–524. - PubMed
-
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J (2000). Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol 164:2–14. - PubMed
-
- Bartlett JS, Samulski RJ, McCown TJ (1998). Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum Gene Ther 9:1181–1186. - PubMed
-
- Beaun D, Hagenzieker J, Jack R (2004). Neuronal ceroid lipofuscinosis: what are the roles of electron microscopy, DNA, and enzyme analysis in diagnosis? J Histotechnol 27:237–243.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
