The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging
- PMID: 16452664
- PMCID: PMC6675495
- DOI: 10.1523/JNEUROSCI.3463-05.2006
The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging
Abstract
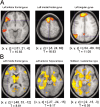
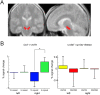
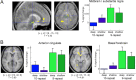
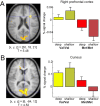
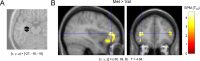
Recent data from animal studies raise the possibility that dopaminergic neuromodulation promotes the encoding of novel stimuli. We investigated a possible role for the dopaminergic midbrain in human episodic memory by measuring how polymorphisms in dopamine clearance pathways affect encoding-related brain activity (functional magnetic resonance imaging) in an episodic memory task. In 51 young, healthy adults, successful episodic encoding was associated with activation of the substantia nigra. This midbrain activation was modulated by a functional variable number of tandem repeat (VNTR) polymorphism in the dopamine transporter (DAT1) gene. Despite no differences in memory performance between genotype groups, carriers of the (low expressing) 9-repeat allele of the DAT1 VNTR showed relatively higher midbrain activation when compared with subjects homozygous for the 10-repeat allele, who express DAT1 at higher levels. The catechol-O-methyl transferase (COMT) Val108/158Met polymorphism, which is known to modulate enzyme activity, affected encoding-related activity in the right prefrontal cortex (PFC) and in occipital brain regions but not in the midbrain. Moreover, subjects homozygous for the (low activity) Met allele showed stronger functional coupling between the PFC and the hippocampus during encoding. Our finding that genetic variations in the dopamine clearance pathways affect encoding-related activation patterns in midbrain and PFC provides strong support for a role of dopaminergic neuromodulation in human episodic memory formation. It also supports the hypothesis of anatomically and functionally distinct roles for DAT1 and COMT in dopamine metabolism, with DAT1 modulating rapid, phasic midbrain activity and COMT being particularly involved in prefrontal dopamine clearance.
Figures
References
-
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP (2004). Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. NeuroImage 23:1460–1471. - PubMed
-
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER (1999). Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA 96:5280–5285. - PMC - PubMed
-
- Barcelo F, Suwazono S, Knight RT (2000). Prefrontal modulation of visual processing in humans. Nat Neurosci 3:399–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
