Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents
- PMID: 16452667
- PMCID: PMC6675481
- DOI: 10.1523/JNEUROSCI.2219-05.2006
Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents
Abstract
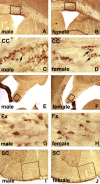
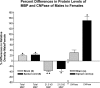
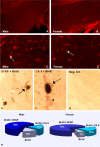
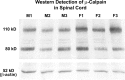
Sexual dimorphism of neurons and astrocytes has been demonstrated in different centers of the brain, but sexual dimorphism of oligodendrocytes and myelin has not been examined. We show, using immunocytochemistry and in situ hybridization, that the density of oligodendrocytes in corpus callosum, fornix, and spinal cord is 20-40% greater in males compared with females. These differences are present in young and aged rodents and are independent of strain and species. Proteolipid protein and carbonic anhydrase-II transcripts, measured by real-time PCR, are approximately two to three times greater in males. Myelin basic protein and 2', 3'-cyclic nucleotide 3'-phosphodiesterase, measured by Western blots, are 20-160% greater in males compared with females. Surprisingly, both generation of new glia and apoptosis of glia, including oligodendrocytes, are approximately two times greater in female corpus callosum. These results indicate that the lifespan of oligodendrocytes is shorter in females than in males. Castration of males produces a female phenotype characterized by fewer oligodendrocytes and increased generation of new glia. These findings indicate that exogenous androgens differentially affect the lifespan of male and female oligodendrocytes, and they can override the endogenous production of neurosteroids. The data imply that turnover of myelin is greater in females than in males. Mu-calpain, a protease upregulated in degeneration of myelin, is dramatically increased at both transcriptional and translational levels in females compared with males. These morphological, molecular, and biochemical data show surprisingly large differences in turnover of oligodendrocytes and myelin between sexes. We discuss the potential significance of these differences to multiple sclerosis, a sexually dimorphic disease, whose progression is altered by exogenous hormones.
Figures




References
-
- Alonso G (2000). Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia 31:219–231. - PubMed
-
- Andrade JP, Madeira MD, Paula-Barbosa MM (2000). Sexual dimorphism in the subiculum of the rat hippocampal formation. Brain Res 875:125–137. - PubMed
-
- Bansal R, Stefansson K, Pfeiffer SE (1992). Proligodendroblast antigen (POA), a developmental antigen expressed by AOO7/O4-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. J Neurochem 58:2221–2229. - PubMed
-
- Barrera A, Jimenez L, Gonzalez GM, Montiel J, Aboitiz F (2001). Dendritic structure of single hippocampal neurons according to sex and hemisphere of origin in middle-aged and elderly human subjects. Brain Res 906:31–37. - PubMed
-
- Bebo BF Jr, Vandenbark AA, Offner H (1996). Male SJL mice do not relapse after induction of EAE with PLP 139–151. J Neurosci Res 45:680–689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
