The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption
- PMID: 16452669
- PMCID: PMC6675490
- DOI: 10.1523/JNEUROSCI.3786-05.2006
The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption
Abstract
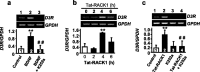
We recently identified a homeostatic pathway that inhibits ethanol intake. This protective pathway consists of the scaffolding protein RACK1 and brain-derived neurotrophic factor (BDNF). RACK1 translocates to the nucleus after exposure of neurons to ethanol and increases expression of BDNF (McGough et al., 2004). We also found that increasing the levels of BDNF via systemic administration of RACK1 expressed as a Tat-fusion protein (Tat-RACK1) reduces ethanol consumption, whereas reduction of BDNF levels augments this behavior (McGough et al., 2004). Based on these results, we hypothesized that activation of the BDNF receptor TrkB is necessary for the effects of BDNF on ethanol intake and that gene products downstream of BDNF negatively regulate ethanol consumption. Here, we show that inhibition of the BDNF receptor TrkB increases voluntary ethanol consumption in wild-type mice but not in mice lacking one copy of the BDNF gene (BDNF(+/-)). We also find that increases in the levels of BDNF, mediated by ethanol or RACK1, lead to increased dorsal striatal levels of the dopamine D3 receptor (D3R), a gene downstream of BDNF, via activation of the TrkB receptor. Finally, we show that the Tat-RACK1-mediated reduction of ethanol consumption is attenuated by coinjection with either the Trk inhibitor K252a or the dopamine D3R-prefering antagonist U-99194A [5, 6-dimethoxy-2-(di-n-propylamino)indan], suggesting that activation of the BDNF pathway via RACK1 leads to increased expression of the dopamine D3R, which in turn mediates the attenuation of ethanol consumption.
Figures



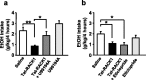
Similar articles
-
Dopamine D3 receptor is necessary for ethanol consumption: an approach with buspirone.Neuropsychopharmacology. 2014 Jul;39(8):2017-28. doi: 10.1038/npp.2014.51. Epub 2014 Mar 3. Neuropsychopharmacology. 2014. PMID: 24584330 Free PMC article.
-
RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction.J Neurosci. 2004 Nov 17;24(46):10542-52. doi: 10.1523/JNEUROSCI.3714-04.2004. J Neurosci. 2004. PMID: 15548669 Free PMC article.
-
Brain-derived neurotrophic factor mediates the suppression of alcohol self-administration by memantine.Addict Biol. 2014 Sep;19(5):758-69. doi: 10.1111/adb.12039. Epub 2013 Feb 16. Addict Biol. 2014. PMID: 23414063
-
Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake.FASEB J. 2008 Jul;22(7):2393-404. doi: 10.1096/fj.07-099135. Epub 2008 Feb 29. FASEB J. 2008. PMID: 18310464
-
Ethanol-BDNF interactions: still more questions than answers.Pharmacol Ther. 2008 Apr;118(1):36-57. doi: 10.1016/j.pharmthera.2008.01.003. Epub 2008 Feb 2. Pharmacol Ther. 2008. PMID: 18394710 Free PMC article. Review.
Cited by
-
BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis.Eur J Neurosci. 2013 Feb;37(4):607-12. doi: 10.1111/ejn.12067. Epub 2012 Nov 28. Eur J Neurosci. 2013. PMID: 23189980 Free PMC article.
-
Increasing Brain-Derived Neurotrophic Factor (BDNF) in medial prefrontal cortex selectively reduces excessive drinking in ethanol dependent mice.Neuropharmacology. 2018 Sep 15;140:35-42. doi: 10.1016/j.neuropharm.2018.07.031. Epub 2018 Jul 26. Neuropharmacology. 2018. PMID: 30056122 Free PMC article.
-
Dopamine D3 receptor is necessary for ethanol consumption: an approach with buspirone.Neuropsychopharmacology. 2014 Jul;39(8):2017-28. doi: 10.1038/npp.2014.51. Epub 2014 Mar 3. Neuropsychopharmacology. 2014. PMID: 24584330 Free PMC article.
-
Prenatal and postnatal ethanol experiences modulate consumption of the drug in rat pups, without impairment in the granular cell layer of the main olfactory bulb.Physiol Behav. 2011 Jan 10;102(1):63-75. doi: 10.1016/j.physbeh.2010.10.009. Epub 2010 Oct 15. Physiol Behav. 2011. PMID: 20951715 Free PMC article.
-
Higher levels of D2R and D3R in the frontal-striatal regions are associated with reduced perseverative reward seeking after opioid abstinence.Front Behav Neurosci. 2025 Jun 2;19:1552055. doi: 10.3389/fnbeh.2025.1552055. eCollection 2025. Front Behav Neurosci. 2025. PMID: 40529937 Free PMC article.
References
-
- Audinot V, Newman-Tancredi A, Gobert A, Rivet JM, Brocco M, Lejeune F, Gluck L, Desposte I, Bervoets K, Dekeyne A, Millan MJ (1998). A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (+)-S 14297, nafadotride, GR 103, 691 and U 99194. J Pharmacol Exp Ther 287:187–197. - PubMed
-
- Blanchard BA, Steindorf S, Wang S, Glick SD (1993). Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res 17:968–973. - PubMed
-
- Boulay D, Depoortere R, Rostene W, Perrault G, Sanger DJ (1999). Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock-out and wild-type mice. Neuropharmacology 38:555–565. - PubMed
-
- Boyce JM, Risinger FO (2000). Enhancement of ethanol reward by dopamine D3 receptor blockade. Brain Res 880:202–206. - PubMed
-
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM (2001). Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther 297:27–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
