Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine beta hydroxlyase mutation
- PMID: 16452672
- PMCID: PMC2673197
- DOI: 10.1523/JNEUROSCI.4749-05.2006
Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine beta hydroxlyase mutation
Abstract
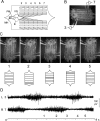
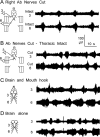
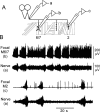
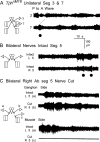
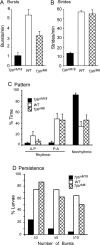
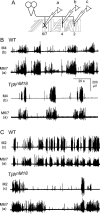
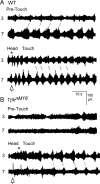
Forward locomotion of Drosophila melanogaster larvae is composed of rhythmic waves of contractions that are thought to be produced by segmentally organized central pattern generators. We present a systematic description of spike activity patterns during locomotive contraction waves in semi-intact wild-type and mutant larval preparations. We have shown previously that Tbetah(nM18) mutants, with altered levels of octopamine and tyramine, have a locomotion deficit. By recording en passant from the segmental nerves, we investigated the coordination of the neuronal activity driving contraction waves of the abdominal body-wall muscles. Rhythmic bursts of activity that occurred concurrently with locomotive waves were frequently observed in wild-type larvae but were rarely seen in Tbetah(nM18) mutants. These centrally generated patterned activities were eliminated in the distal stumps of both wild-type and Tbetah(nM18) larvae after severing the segmental nerve from the CNS. Patterned activities persisted in the proximal stumps deprived of sensory feedback from the periphery. Simultaneous recordings demonstrated a delay in the bursting activity between different segments, with greater delay for segments that were farther apart. In contrast, bilateral recordings within a single segment revealed a well synchronized activity pattern in nerves innervating each hemisegment in both wild-type and Tbetah(nM18) larvae. Significantly, rhythmic patterns of bursts and waves could be evoked in Tbetah(nM18) mutants by head or tail stimulation despite their highly irregular spontaneous activities. These observations suggest a role of the biogenic amines in the initiation and modulation of motor pattern generation. The technique presented here can be readily extended to examine the locomotion motor program of other mutants.
Figures




References
-
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR (2005). Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46:247–260. - PubMed
-
- Baier A, Wittek B, Brembs B (2002). Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol 205:1233–1240. - PubMed
-
- Barclay JW, Atwood HL, Robertson RM (2002). Impairment of central pattern generation in Drosophila cysteine string protein mutants. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 188:71–78. - PubMed
-
- Belanger JH, Trimmer BA (2000). Combined kinematic and electromyographic analyses of proleg function during crawling by the caterpillar Manduca sexta. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 186:1031–1039. - PubMed
-
- Berrigan D, Pepin DJ (1995). How maggots move: allometry and kinematics of crawling in larval diptera. J Insect Physiol 41:329–337.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
