Delta subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of alpha4beta2delta GABAA receptors
- PMID: 16452673
- PMCID: PMC6675478
- DOI: 10.1523/JNEUROSCI.2913-05.2006
Delta subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of alpha4beta2delta GABAA receptors
Abstract
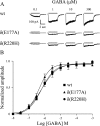
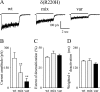
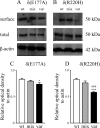
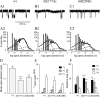
Most human idiopathic generalized epilepsies (IGEs) are polygenic, but virtually nothing is known of the molecular basis for any of the complex epilepsies. Recently, two GABAA receptor delta subunit variants (E177A, R220H) were proposed as susceptibility alleles for generalized epilepsy with febrile seizures plus and juvenile myoclonic epilepsy. In human embryonic kidney 293T cells, recombinant halpha1beta2delta(E177A) and halpha1beta2delta(R220H) receptor currents were reduced, but the basis for the current reduction was not determined. We examined the mechanistic basis for the current reduction produced by these variants using the halpha4beta2delta receptor, an isoform more physiologically relevant and linked to epileptogenesis, by characterizing the effects of these variants on receptor cell surface expression and single-channel gating properties. Expression of variant alpha4beta2delta(R220H) receptors resulted in a decrease in surface receptor proteins, and a smaller, but significant, reduction was observed for variant alpha4beta2delta(E177A) receptors. For both variants, no significant alterations of surface expression were observed for mixed population of wild-type and variant receptors. The mean open durations of alpha4beta2delta(E177A) and alpha4beta2delta(R220H) receptor single-channel currents were both significantly decreased compared to wild-type receptors. These data suggest that both delta(E177A) and delta(R220H) variants may result in disinhibition in IGEs by similar cellular and molecular mechanisms, and in heterozygously affected individuals, a reduction in channel open duration of delta subunit-containing GABAA receptors may be the major contributor to the epilepsy phenotypes.
Figures

References
-
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E (2001). First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nat Genet 28:46–48. - PubMed
-
- Berkovic SF, Howell RA, Hay DA, Hopper JL (1998). Epilepsies in twins: genetics of the major epilepsy syndromes. Ann Neurol 43:435–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
