Simultaneous release of glutamate and acetylcholine from single magnocellular "cholinergic" basal forebrain neurons
- PMID: 16452682
- PMCID: PMC6675485
- DOI: 10.1523/JNEUROSCI.3979-05.2006
Simultaneous release of glutamate and acetylcholine from single magnocellular "cholinergic" basal forebrain neurons
Abstract
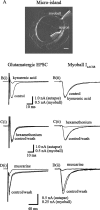
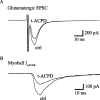
Basal forebrain (BF) neurons provide the principal cholinergic drive to the hippocampus and cortex. Their degeneration is associated with the cognitive defects of Alzheimer's disease. Immunohistochemical studies suggest that some of these neurons contain glutamate, so might also release it. To test this, we made microisland cultures of single BF neurons from 12- to 14-d-old rats. Over 1-8 weeks in culture, neuronal processes made autaptic connections onto the neuron. In 34 of 36 cells tested, a somatically generated action potential was followed by a short-latency EPSC that was blocked by 1 mM kynurenic acid, showing that they released glutamate. To test whether the same neuron also released acetylcholine, we placed a voltage-clamped rat myoball expressing nicotinic receptors in contact with a neurite. In six of six neurons tested, the glutamatergic EPSC was accompanied by a nicotinic (hexamethonium-sensitive) myoball current. Stimulation of the M2-muscarinic presynaptic receptors (characterized using tripitramine and pirenzepine) produced a parallel inhibition of autaptic glutamatergic and myoball nicotinic responses; metabotropic glutamate receptor stimulation produced similar but less consistent and weaker effects. Atropine enhanced the glutamatergic EPSCs during repetitive stimulation by 25 +/- 6%; the anti-cholinesterase neostigmine reduced the train EPSCs by 37 +/- 6%. Hence, synaptically released acetylcholine exerted a negative-feedback inhibition of coreleased glutamate. We conclude that most cholinergic basal forebrain neurons are capable of releasing glutamate as a cotransmitter and that the release of both transmitters is subject to simultaneous feedback inhibition by synaptically released acetylcholine. This has implications for BF neuron function and for the use of cholinesterase inhibitors in Alzheimer's disease.
Figures




References
-
- Beaulieu C, Somogyi P (1991). Enrichment of cholinergic synaptic terminals on GABAergic neurons and coexistence of immunoreactive GABA and choline acetyltransferase in the same synaptic terminals in the striate cortex of the cat. J Comp Neurol 304:666–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
