The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments
- PMID: 16452683
- PMCID: PMC2638122
- DOI: 10.1523/JNEUROSCI.4946-05.2006
The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments
Abstract
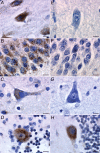
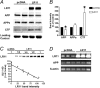
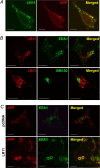
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by progressive cognitive decline and neuropathological changes, including the deposition of amyloid beta (Abeta) in senile plaques. The mechanisms causing the disease and Abeta accumulation are not well understood, but important genetic associations with apolipoprotein E genotype and involvement of lipoprotein receptors have become apparent. LR11 (also known as SorLA), a member of the low-density lipoprotein receptor family, has been identified previously as an altered transcript in microarray analyses of samples from human AD cases. Here, we show neuronal expression of the lipoprotein receptor LR11 in control brain in regions vulnerable to AD neuropathology and marked reduction of LR11 expression in these regions in AD brains before cell death. Overexpression of LR11 drastically reduces levels of extracellular Abeta and also lowers levels of total cellular amyloid precursor protein (APP). LR11 colocalizes with APP and regulates its trafficking in endocytic compartments, which are important intracellular sites for APP processing and Abeta generation. Endogenous LR11 localizes to neuronal multivesicular bodies in both rat and human brain. The robust correlation between reduced LR11 expression and AD neuropathology and its potent effects on extracellular Abeta levels suggest that this neuronal lipoprotein receptor could play an important role in AD pathogenesis.
Figures

References
-
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE (2005). Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci USA 102:13461–13466. - PMC - PubMed
-
- Ceresa BP, Lotscher M, Schmid SL (2001). Receptor and membrane recycling can occur with unaltered efficiency despite dramatic Rab5(q79l)-induced changes in endosome geometry. J Biol Chem 276:9649–9654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
