Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals
- PMID: 16453019
- PMCID: PMC1359039
- DOI: 10.1172/JCI25167
Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals
Abstract
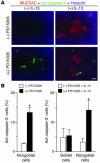
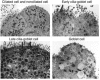
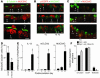
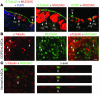
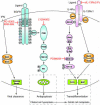
Epithelial hyperplasia and metaplasia are common features of inflammatory and neoplastic disease, but the basis for the altered epithelial phenotype is often uncertain. Here we show that long-term ciliated cell hyperplasia coincides with mucous (goblet) cell metaplasia after respiratory viral clearance in mouse airways. This chronic switch in epithelial behavior exhibits genetic susceptibility and depends on persistent activation of EGFR signaling to PI3K that prevents apoptosis of ciliated cells and on IL-13 signaling that promotes transdifferentiation of ciliated to goblet cells. Thus, EGFR blockade (using an irreversible EGFR kinase inhibitor designated EKB-569) prevents virus-induced increases in ciliated and goblet cells whereas IL-13 blockade (using s-IL-13Ralpha2-Fc) exacerbates ciliated cell hyperplasia but still inhibits goblet cell metaplasia. The distinct effects of EGFR and IL-13 inhibitors after viral reprogramming suggest that these combined therapeutic strategies may also correct epithelial architecture in the setting of airway inflammatory disorders characterized by a similar pattern of chronic EGFR activation, IL-13 expression, and ciliated-to-goblet cell metaplasia.
Figures

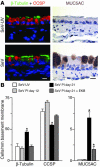
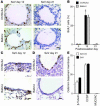
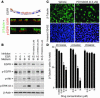
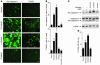
Comment in
-
Mucus in chronic airway diseases: sorting out the sticky details.J Clin Invest. 2006 Feb;116(2):306-8. doi: 10.1172/JCI27690. J Clin Invest. 2006. PMID: 16453018 Free PMC article.
Similar articles
-
Role of epidermal growth factor receptor in maintaining airway goblet cell hyperplasia in rats sensitized to allergen.Clin Exp Allergy. 2008 May;38(5):857-65. doi: 10.1111/j.1365-2222.2008.02951.x. Epub 2008 Feb 26. Clin Exp Allergy. 2008. PMID: 18307528
-
Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium.Am J Respir Cell Mol Biol. 2006 May;34(5):581-91. doi: 10.1165/rcmb.2005-0386OC. Epub 2006 Jan 19. Am J Respir Cell Mol Biol. 2006. PMID: 16424381 Free PMC article.
-
Involvement of the p38 MAPK pathway in IL-13-induced mucous cell metaplasia in mouse tracheal epithelial cells.Respirology. 2008 Mar;13(2):191-202. doi: 10.1111/j.1440-1843.2008.01237.x. Respirology. 2008. PMID: 18339016
-
Regulation of mucin expression in respiratory diseases.Biochem Soc Trans. 2009 Aug;37(Pt 4):877-81. doi: 10.1042/BST0370877. Biochem Soc Trans. 2009. PMID: 19614611 Review.
-
Cellular and molecular mechanisms of goblet cell metaplasia in the respiratory airways.Exp Lung Res. 2013 May-Jun;39(4-5):207-16. doi: 10.3109/01902148.2013.791733. Epub 2013 May 3. Exp Lung Res. 2013. PMID: 23638644 Review.
Cited by
-
Putting the Squeeze on Airway Epithelia.Physiology (Bethesda). 2015 Jul;30(4):293-303. doi: 10.1152/physiol.00004.2015. Physiology (Bethesda). 2015. PMID: 26136543 Free PMC article. Review.
-
HSP90 inhibitor geldanamycin reverts IL-13- and IL-17-induced airway goblet cell metaplasia.J Clin Invest. 2019 Feb 1;129(2):744-758. doi: 10.1172/JCI123524. Epub 2019 Jan 14. J Clin Invest. 2019. PMID: 30640172 Free PMC article.
-
Nuclear factor-kappaB activation in airway epithelium induces inflammation and hyperresponsiveness.Am J Respir Crit Care Med. 2008 May 1;177(9):959-69. doi: 10.1164/rccm.200707-1096OC. Epub 2008 Feb 8. Am J Respir Crit Care Med. 2008. PMID: 18263801 Free PMC article.
-
Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma.Curr Mol Med. 2008 Aug;8(5):384-92. doi: 10.2174/156652408785161032. Curr Mol Med. 2008. PMID: 18691065 Free PMC article. Review.
-
Chronic Obstructive Pulmonary Disease Is Not Associated with KRAS Mutations in Non-Small Cell Lung Cancer.PLoS One. 2016 Mar 23;11(3):e0152317. doi: 10.1371/journal.pone.0152317. eCollection 2016. PLoS One. 2016. PMID: 27008036 Free PMC article.
References
-
- Wills-Karp M, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. - PubMed
-
- Kondo M, Tamaoki J, Takeyama K, Nakata J, Nagai J. Interleukin-13 induces goblet cell differentiation in primary cell culture from guinea pig tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 2002;27:536–541. - PubMed
-
- Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L730–L739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

