GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway
- PMID: 16453021
- PMCID: PMC1359053
- DOI: 10.1172/JCI26295
GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway
Abstract
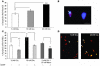
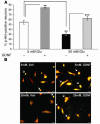
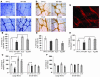
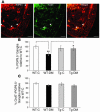
Diabetes can result in loss of enteric neurons and subsequent gastrointestinal complications. The mechanism of enteric neuronal loss in diabetes is not known. We examined the effects of hyperglycemia on enteric neuronal survival and the effects of glial cell line-derived neurotrophic factor (GDNF) on modulating this survival. Exposure of primary enteric neurons to 20 mM glucose (hyperglycemia) for 24 hours resulted in a significant increase in apoptosis compared with 5 mM glucose (normoglycemia). Exposure to 20 mM glucose resulted in decreased Akt phosphorylation and enhanced nuclear translocation of forkhead box O3a (FOXO3a). Treatment of enteric neurons with GDNF ameliorated these changes. In streptozotocin-induced diabetic mice, there was evidence of myenteric neuronal apoptosis and reduced Akt phosphorylation. Diabetic mice had loss of NADPH diaphorase-stained myenteric neurons, delayed gastric emptying, and increased intestinal transit time. The pathophysiological effects of hyperglycemia (apoptosis, reduced Akt phosphorylation, loss of inhibitory neurons, motility changes) were reversed in diabetic glial fibrillary acidic protein-GDNF (GFAP-GDNF) Tg mice. In conclusion, we demonstrate that hyperglycemia induces neuronal loss through a reduction in Akt-mediated survival signaling and that these effects are reversed by GDNF. GDNF may be a potential therapeutic target for the gastrointestinal motility disorders related to diabetes.
Figures







Comment in
-
Gastrointestinal motility and glycemic control in diabetes: the chicken and the egg revisited?J Clin Invest. 2006 Feb;116(2):299-302. doi: 10.1172/JCI27758. J Clin Invest. 2006. PMID: 16453015 Free PMC article. Review.
References
-
- Harris MI, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–524. - PubMed
-
- Mokdad AH, et al. The continuing increase of diabetes in the US [letter] Diabetes Care. 2001;24:412. - PubMed
-
- Goyal RK, Spiro HM. Gastrointestinal manifestations of diabetes mellitus. Med. Clin. North Am. 1971;55:1031–1044. - PubMed
-
- Verne G, Sninsky CA. Diabetes and the gastrointestinal tract. Gastroenterol. Clin. North Am. 1998;27:861–874. - PubMed
-
- Schmidt RE, Plurad SB, Modert CW. Experimental diabetic autonomic neuropathy characterization in streptozotocin-diabetic Sprague-Dawley rats. Lab. Invest. 1983;49:538–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

