Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers
- PMID: 16453022
- PMCID: PMC1359048
- DOI: 10.1172/JCI25843
Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers
Abstract
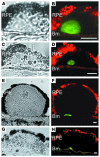
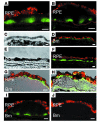
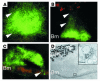
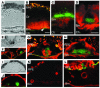
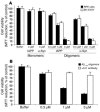
Protein misfolding and aggregation are thought to underlie the pathogenesis of many amyloid diseases, such as Alzheimer and Parkinson diseases, whereby a stepwise protein misfolding process begins with the conversion of soluble protein monomers to prefibrillar oligomers and progresses to the formation of insoluble amyloid fibrils. Drusen are extracellular deposits found in aging eyes and in eyes afflicted with age-related macular degeneration (AMD). Recent characterizations of drusen have revealed protein components that are shared with amyloid deposits. However, characteristic amyloid fibrils have thus far not been identified in drusen. In this study, we tested the hypothesis that nonfibrillar oligomers may be a common link in amyloid diseases. Oligomers consisting of distinct amyloidogenic proteins and peptides can be detected by a recently developed antibody that is thought to recognize a common structure. Notably, oligomers exhibit cellular toxicity, which suggests that they play a role in the pathogenesis of neurodegenerative diseases. Through use of the anti-oligomer antibody, we came to observe the presence of nonfibrillar, toxic oligomers in drusen. Conversely, no reactivity was observed in age-matched control eyes without drusen. These results suggest that amyloid oligomers may be involved in drusen biogenesis and that similar protein misfolding processes may occur in AMD and amyloid diseases.
Figures
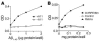
References
-
- Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am. J. Ophthalmol. 2004;137:486–495. - PubMed
-
- Johnson PT, et al. Drusen-associated degeneration in the retina. Invest. Ophthalmol. Vis. Sci. 2003;44:4481–4488. - PubMed
-
- Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv. Ophthalmol. 1999;44:1–29. - PubMed
-
- Anderson DH, et al. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp. Eye Res. 2004;78:243–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

