The immunologically active site of prothymosin alpha is located at the carboxy-terminus of the polypeptide. Evaluation of its in vitro effects in cancer patients
- PMID: 16453152
- PMCID: PMC11030181
- DOI: 10.1007/s00262-005-0108-4
The immunologically active site of prothymosin alpha is located at the carboxy-terminus of the polypeptide. Evaluation of its in vitro effects in cancer patients
Abstract
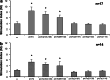
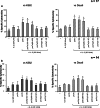
Prothymosin alpha (proTalpha) is a 109 amino acid long polypeptide presenting distinct immunoenhancing activity in vitro and in vivo. Recent reports suggest that in apoptotic cells, proTalpha is cleaved by caspases at its carboxy(C)-terminus generating potentially bioactive fragments. In this study, we identified the peptide segment of proTalpha presenting maximum immunomodulatory activity. Calf thymus proTalpha was trypsinised, and the five fragments produced (spanning residues 1-14, 21-30, 31-87, 89-102 and 103-109) were tested for their ability to stimulate healthy donor- and cancer patient-derived peripheral blood mononuclear cell (PBMC) proliferation in autologous mixed lymphocyte reaction (AMLR), natural killer and lymphokine-activated killer cell activity, intracellular production of perforin, upregulation of adhesion molecules and CD25 expression. ProTalpha(89-102) and proTalpha(103-109) significantly fortified healthy donor-lymphocytes' immune responses to levels comparable to those induced by intact proTalpha. These effects were more pronounced in cancer patients, where peptides proTalpha(89-102) and proTalpha(103-109) partly, however significantly, restored the depressed AMLR and cytolytic ability of PBMC, by simulating the biological activity exerted by intact proTalpha. ProTalpha(1-14), proTalpha(21-30) and proTalpha(31-87) marginally upregulated lymphocyte activation. This is the first report showing that proTalpha's immunomodulating activity can be substituted by its C-terminal peptide(s). Whether generation and externalization of such immunoactive proTalpha fragments occurs in vivo, needs further investigation. However, if these peptides can trigger immune responses, they may eventually be used therapeutically to improve some PBMC functions of cancer patients.
Figures

References
-
- Haritos AA. α-Thymosins: relationships in structure, distribution, and function. Isozymes Curr Top Biol Med Res. 1987;14:123–152. - PubMed
-
- Tsitsiloni OE, Stiakakis J, Koutselinis A, Gogas J, Markopoulos C, Yialouris P, Bekris S, Panoussopoulos D, Kiortsis V, Voelter W, Haritos AA. Expression of α-thymosins in human tissues in normal and abnormal growth. Proc Natl Acad Sci USA. 1993;90:9504–9507. doi: 10.1073/pnas.90.20.9504. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

