Efficient neutralization of anthrax toxin by chimpanzee monoclonal antibodies against protective antigen
- PMID: 16453257
- PMCID: PMC7110013
- DOI: 10.1086/500148
Efficient neutralization of anthrax toxin by chimpanzee monoclonal antibodies against protective antigen
Abstract
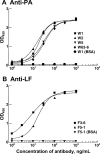
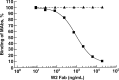
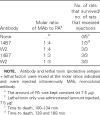
Four single-chain variable fragments (scFvs) against protective antigen (PA) and 2 scFvs against lethal factor (LF) of anthrax were isolated from a phage display library generated from immunized chimpanzees. Only 2 scFvs recognizing PA (W1 and W2) neutralized the cytotoxicity of lethal toxin in a macrophage lysis assay. Full-length immunoglobulin G (IgG) of W1 and W2 efficiently protected rats from anthrax toxin challenge. The epitope recognized by W1 and W2 was conformational and was formed by C-terminal amino acids 614-735 of PA. W1 and W2 each bound to PA with an equilibrium dissociation constant of 4x10-11 mol/L to 5x10(-11) mol/L, which is an affinity that is 20-100-fold higher than that for the interaction of the receptor and PA. W1 and W2 inhibited the binding of PA to the receptor, suggesting that this was the mechanism of protection. These data suggest that W1 and W2 chimpanzee monoclonal antibodies may serve as PA entry inhibitors for use in the emergency prophylaxis against and treatment of anthrax.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

