Comparison of serum hepatitis C virus (HCV) RNA and core antigen levels in patients coinfected with human immunodeficiency virus and HCV and treated with interferon plus ribavirin
- PMID: 16455894
- PMCID: PMC1392678
- DOI: 10.1128/JCM.44.2.417-422.2006
Comparison of serum hepatitis C virus (HCV) RNA and core antigen levels in patients coinfected with human immunodeficiency virus and HCV and treated with interferon plus ribavirin
Abstract
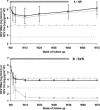
Trak-C (Ortho-Clinical Diagnostics) is an enzyme-linked immunosorbent assay-based method capable of quantifying hepatitis C virus (HCV) core antigen (CA) in serum and could be an alternative to molecular detection and quantification of HCV RNA. We have evaluated the Trak-C assay in comparison with an HCV RNA quantitative assay (Versant HCV v3.0; Bayer Diagnostics) in the follow-up of 348 treated, human immunodeficiency virus (HIV)/HCV-coinfected patients included in the ANRS HC02 RIBAVIC trial. ANRS HC02 RIBAVIC is a therapeutic, multicenter, randomized protocol comparing the efficacy of alpha interferon 2b (IFN-alpha2b) (3 million units three times a week)-ribavirin (800 mg/day) to that of pegylated IFN-alpha2b (1.5 mug/kg of body weight/week)-ribavirin (800 mg/day) during 48 weeks of treatment of HIV/HCV-coinfected patients naïve to HCV treatment. Patients were assessed for virological analysis at day 0 and weeks 4, 12, 24, 48, and 72. Correlation of HCV RNA and HCV CA at the initiation of treatment was excellent (r = 0.92). HCV RNA and CA kinetics were similar during follow-up of HCV treatment from day 0 to week 72 whatever the group of response and genotype. The positive and negative predictive values of response to the treatment at week 4 were 59 and 94%, respectively, for HCV RNA load reduction of >2 log and 54 and 94%, respectively, for HCV CA below the threshold value (4.18 log(10) pg/ml . 10(4)). Trak-C, a new assay able to quantify CA in HIV/HCV-coinfected patients, correlates well with quantitative HCV RNA assays and is cheaper and easier to perform than molecular technology. HCV CA could be a valuable alternative test for therapeutic follow-up of coinfected patients treated with IFN plus ribavirin in developing countries.
Figures



Similar articles
-
[Comparison of hepatitis C viral RNA and core antigen kinetics in the therapeutic follow up of hepatitis C virus and human immunodeficiency virus co-infected patients, treated by bitherapy interferon-ribavirin, within the framework of RIBAVIC protocol].Pathol Biol (Paris). 2004 Nov;52(9):522-8. doi: 10.1016/j.patbio.2004.07.036. Pathol Biol (Paris). 2004. PMID: 15531116 French.
-
Critical role of ribavirin for the achievement of early virological response to HCV therapy in HCV/HIV-coinfected patients.J Viral Hepat. 2007 Jun;14(6):387-91. doi: 10.1111/j.1365-2893.2006.00806.x. J Viral Hepat. 2007. PMID: 17501758 Clinical Trial.
-
The predictive value of core antigen testing for the management of hepatitis C patients receiving pegylated interferon/ribavirin treatment.J Med Virol. 2004 Jul;73(3):392-6. doi: 10.1002/jmv.20104. J Med Virol. 2004. PMID: 15170634
-
HIV and hepatitis C co-infection: acute HCV therapy.Curr Opin HIV AIDS. 2011 Nov;6(6):459-64. doi: 10.1097/COH.0b013e32834b87de. Curr Opin HIV AIDS. 2011. PMID: 22001891 Review.
-
Pegylated IFN-alpha2b plus ribavirin for treatment-naive patients coinfected with HCV and HIV.Expert Rev Anti Infect Ther. 2008 Jun;6(3):281-9. doi: 10.1586/14787210.6.3.281. Expert Rev Anti Infect Ther. 2008. PMID: 18588492 Review.
Cited by
-
Antiviral treatment for chronic hepatitis C in patients with human immunodeficiency virus.Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD004888. doi: 10.1002/14651858.CD004888.pub2. Cochrane Database Syst Rev. 2010. PMID: 20091566 Free PMC article.
-
HIV-1 Nef interacts with HCV Core, recruits TRAF2, TRAF5 and TRAF6, and stimulates HIV-1 replication in macrophages.J Innate Immun. 2013;5(6):639-56. doi: 10.1159/000350517. Epub 2013 Jun 13. J Innate Immun. 2013. PMID: 23774506 Free PMC article.
References
-
- Agence Nationale d'Accreditation et d'Evalution en Sante. 2002. Consensus conference: treatment of hepatitis C. Gastroenterol. Clin. Biol. 26:B303-B320. - PubMed
-
- Berg, T., C. Sarrazin, E. Herrman, H. Hinrichsen, T. Gerlach, R. Zachoval, B. Wiedenmann, U. Hopf, and S. Zeuzem. 2003. Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology 37:600-609. - PubMed
-
- Carrat, F., F. Bani-Sadr, S. Pol, E. Rosenthal, F. Lunel-Fabiani, A. Benzekri, P. Morand, C. Goujard, G. Pialoux, L. Piroth, D. Salmon-Ceron, C. Degott, P. Cacoub, C. Perronne, et al. 2004. Pegylated interferon alfa-2b versus standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA 292:2839-2848. - PubMed
-
- Chung, R., J. Andersen, P. Volberding, G. Robbins, T. Liu, K. Sherman, M. Peters, M. Koziel, A. Bahn, B. Alston, D. Colquhoun, T. Nevin, G. Harb, and C. Van der Host. 2004. Pegylated interferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV co-infected persons. N. Engl. J. Med. 351:451-459. - PMC - PubMed
-
- Davis, G. L., J. B. Wong, J. G. McHutchison, M. P. Manns, J. Harvey, and J. Albrecht. 2003. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology 38:645-652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

