Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis
- PMID: 16455920
- PMCID: PMC1392688
- DOI: 10.1128/JCM.44.2.595-597.2006
Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis
Abstract
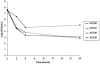
The emergence of a clinically daptomycin-resistant Staphylococcus aureus isolate occurred during treatment of methicillin-resistant S. aureus bacteremia and probable vertebral osteomyelitis. The breakthrough isolate was indistinguishable from pretreatment daptomycin-susceptible isolates by pulsed-field gel electrophoresis. Daptomycin nonsusceptibility was confirmed by MIC and time-kill curve analyses.
Figures

References
-
- Arbeit, R. D., D. Maki, F. P. Tally, E. Campanaro, and B. I. Eisenstein. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673-1681. - PubMed
-
- Carpenter, C. F., and H. F. Chambers. 2004. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin. Infect. Dis. 38:994-1000. - PubMed
-
- Center for Drug Evaluation and Research, Food and Drug Administration. 15. December 2003, posting date. NDA 21-572, CubicinTM (daptomycin for injection). [Online.] http://www.fda.gov/cder/foi/nda/2003/21-572_Cubicin_Microbr_P2.pdf. Accessed 12 June 2005.
-
- Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement, M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
-
- Cubist Pharmaceuticals, Inc. 2003, posting date. CubicinTM (daptomycin for Injection), prescriber information. [Online.] http://www.cubicin.com/documents/cubicin_large_pi.pdf. Accessed 12 June 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

