Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo
- PMID: 16455953
- PMCID: PMC1895285
- DOI: 10.1182/blood-2005-09-3687
Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo
Abstract
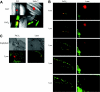
The role of the collagen receptor glycoprotein VI (GPVI) in arteriolar thrombus formation was studied in FcRgamma-null mice (FcRgamma(-/-)) lacking platelet surface GPVI. Thrombi were induced with severe or mild FeCl(3) injury. Collagen exposure was significantly delayed and diminished in mild compared with severe FeCl(3) injury. Times to initial thrombus formation and vessel occlusion were delayed in FcRgamma(-/-) compared with wild-type mice after severe injury. Platelet accumulation in wild-type mice was decreased after mild compared with severe injury. However, there was little difference between platelet accumulation after severe or mild injury in FcRgamma(-/-). These data indicate a significant role for GPVI in FeCl(3)-induced thrombus formation. Pretreatment of wild-type mice with lepirudin further impaired mild FeCl(3)-induced thrombus formation, demonstrating a role for thrombin. Laser-induced thrombus formation in wild-type and FcRgamma(-/-) was comparable. Collagen exposure to circulating blood was undetectable after laser injury. Normalized for thrombus size, thrombus-associated tissue factor was 5-fold higher in laser-induced thrombi than in severe FeCl(3)-induced thrombi. Thus, platelet activation by thrombin appears to be more important after laser injury than platelet activation by GPVI-collagen. It may thus be important when considering targets for antithrombotic therapy to use multiple animal models with diverse pathways to thrombus formation.
Figures

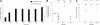
References
-
- Baumgartner HR, Tschopp TB, Weiss HJ. Platelet interaction with collagen fibrils in flowing blood, II: impaired adhesion-aggregation in bleeding disorders: a comparison with subendothelium. Thromb Haemost. 1977;37: 17-28. - PubMed
-
- Meyer D, Baumgartner HR. Role of von Willebrand factor in platelet adhesion to the subendothelium. Br J Haematol. 1983;54: 1-9. - PubMed
-
- Santoro SA. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell. 1986;46: 913-920. - PubMed
-
- Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94: 657-666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

