Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury
- PMID: 16456142
- PMCID: PMC2662912
- DOI: 10.1164/rccm.200509-1477OC
Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury
Abstract
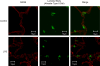
Rationale: Receptor for advanced glycation end-products (RAGE) is one of the alveolar type I cell-associated proteins in the lung.
Objectives: To test the hypothesis that RAGE is a marker of alveolar epithelial type I cell injury.
Methods: Rats were instilled intratracheally with 10 mg/kg lipopolysaccharide or hydrochloric acid. RAGE levels were measured in the bronchoalveolar lavage (BAL) and serum in the rats and in the pulmonary edema fluid and plasma from patients with acute lung injury (ALI; n = 22) and hydrostatic pulmonary edema (n = 11).
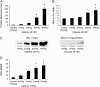
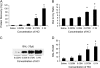
Main results: In the rat lung injury studies, RAGE was released into the BAL and serum as a single soluble isoform sized approximately 48 kD. The elevated levels of RAGE in the BAL correlated well with the severity of experimentally induced lung injury. In the human studies, the RAGE level in the pulmonary edema fluid was significantly higher than the plasma level (p < 0.0001). The median edema fluid/plasma ratio of RAGE levels was 105 (interquartile range, 55-243). The RAGE levels in the pulmonary edema fluid from patients with ALI were higher than the levels from patients with hydrostatic pulmonary edema (p < 0.05), and the plasma RAGE level in patients with ALI were significantly higher than the healthy volunteers (p < 0.001) or patients with hydrostatic pulmonary edema (p < 0.05).
Conclusion: RAGE is a marker of type I alveolar epithelial cell injury based on experimental studies in rats and in patients with ALI.
Figures

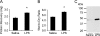



References
-
- Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, Dobbs L. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol 2004;31:309–316. - PubMed
-
- McElroy MC, Kasper M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur Respir J 2004;24:664–673. - PubMed
-
- Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res 1999;84:489–497. - PubMed
-
- Sasaki N, Toki S, Chowei H, Saito T, Nakano N, Hayashi Y, Takeuchi M, Makita Z. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer's disease. Brain Res 2001;888:256–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

