B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK
- PMID: 16456541
- PMCID: PMC1383561
- DOI: 10.1038/sj.emboj.7600980
B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK
Abstract
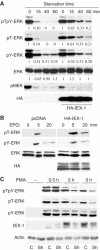
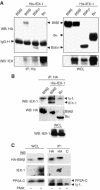
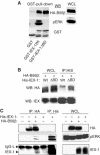
The protein phosphatase 2A (PP2A) acts on several kinases in the extracellular signal-regulated kinase (ERK) signaling pathway but whether a specific holoenzyme dephosphorylates ERK and whether this activity is controlled during mitogenic stimulation is unknown. By using both RNA interference and overexpression of PP2A B regulatory subunits, we show that B56, but not B, family members of PP2A increase ERK dephosphorylation, without affecting its activation by MEK. Induction of the early gene product and ERK substrate IEX-1 (ier3) by growth factors leads to opposite effects and reverses B56-PP2A-mediated ERK dephosphorylation. IEX-1 binds to B56 subunits and pERK independently, enhances B56 phosphorylation by ERK at a conserved Ser/Pro site in this complex and triggers dissociation from the catalytic subunit. This is the first demonstration of the involvement of B56-containing PP2A in ERK dephosphorylation and of a B56-specific cellular protein inhibitor regulating its activity in an ERK-dependent fashion. In addition, our results raise a new paradigm in ERK signaling in which ERK associated to a substrate can transphosphorylate nearby proteins.
Figures
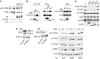




References
-
- Adams DG, Coffee RL Jr, Zhang H, Pelech S, Strack S, Wadzinski BE (2005) Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J Biol Chem 280: 42644–42654 - PubMed
-
- Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P (1995) Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol 5: 283–295 - PubMed
-
- Brondello JM, Pouyssegur J, McKenzie FR (1999) Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286: 2514–2517 - PubMed
-
- Camps M, Nichols A, Gillieron C, Antonsson B, Muda M, Chabert C, Boschert U, Arkinstall S (1998) Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280: 1262–1265 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

