MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration
- PMID: 16456544
- PMCID: PMC1383554
- DOI: 10.1038/sj.emboj.7600973
MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration
Abstract
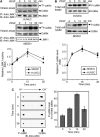
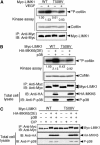
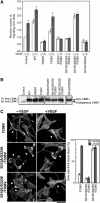
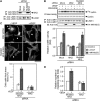
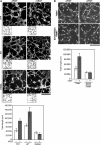
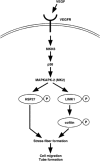
Vascular endothelial growth factor-A (VEGF-A) induces actin reorganization and migration of endothelial cells through a p38 mitogen-activated protein kinase (MAPK) pathway. LIM-kinase 1 (LIMK1) induces actin remodeling by phosphorylating and inactivating cofilin, an actin-depolymerizing factor. In this study, we demonstrate that activation of LIMK1 by MAPKAPK-2 (MK2; a downstream kinase of p38 MAPK) represents a novel signaling pathway in VEGF-A-induced cell migration. VEGF-A induced LIMK1 activation and cofilin phosphorylation, and this was inhibited by the p38 MAPK inhibitor SB203580. Although p38 phosphorylated LIMK1 at Ser-310, it failed to activate LIMK1 directly; however, MK2 activated LIMK1 by phosphorylation at Ser-323. Expression of a Ser-323-non-phosphorylatable mutant of LIMK1 suppressed VEGF-A-induced stress fiber formation and cell migration; however, expression of a Ser-323-phosphorylation-mimic mutant enhanced these processes. Knockdown of MK2 by siRNA suppressed VEGF-A-induced LIMK1 activation, stress fiber formation, and cell migration. Expression of kinase-dead LIMK1 suppressed VEGF-A-induced tubule formation. These findings suggest that MK2-mediated LIMK1 phosphorylation/activation plays an essential role in VEGF-A-induced actin reorganization, migration, and tubule formation of endothelial cells.
Figures




References
-
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stayon CA, Bernard O, Caroni P (1998) Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393: 805–809 - PubMed
-
- Ashida N, Arai H, Yamasaki M, Kita T (2001) Distinct signaling pathways for MCP-1-dependent integrin activation and chemotaxis. J Biol Chem 276: 16555–16560 - PubMed
-
- Bamburg JR, Wiggan OP (2002) ADF/cofilin and actin dynamics in disease. Trends Cell Biol 12: 598–605 - PubMed
-
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 - PubMed
-
- Dawe HR, Minamide LS, Bamburg JR, Cramer LP (2003) ADF/cofilin controls cell polarity during fibroblast migration. Curr Biol 13: 252–257 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

