B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1
- PMID: 16456548
- PMCID: PMC1383545
- DOI: 10.1038/sj.emboj.7600944
B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1
Abstract
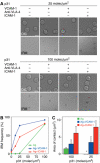
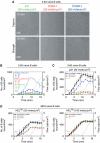
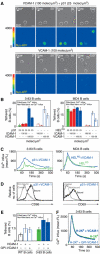
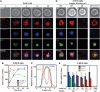
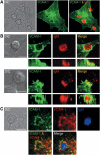
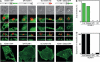
VCAM-1 is one of the main ligands of VLA-4, an integrin that is highly expressed on the surface of mature B cells. Here we find that coexpression of VCAM-1 on an antigen-bearing membrane facilitates B-cell activation. Firstly, this is achieved by mediating B-cell tethering, which in turn increases the likelihood of a B cell to be activated. Secondly, VLA-4 synergizes with the B-cell receptor (BCR), providing B cells with tight adhesion and enhanced signalling. This dual role of VCAM-1 in promoting B-cell activation is predominantly effective when the affinity of the BCR for the antigen is low. In addition, we show that the VCAM-1 ectodomain alone is sufficient to carry out this function. However, it requires the transmembrane domain to segregate properly into a docking structure characteristic of the B-cell immunological synapse (IS). These results show that the VLA-4/VCAM-1 interaction during membrane antigen recognition enhances B-cell activation and this function appears to be independent of its final peripheral localization at the IS.
Figures

References
-
- Barreiro O, Yanez-Mo M, Sala-Valdes M, Gutierrez-Lopez MD, Ovalle S, Higginbottom A, Monk PN, Cabanas C, Sanchez-Madrid F (2005) Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood 105: 2852–2861 - PubMed
-
- Batista FD, Neuberger MS (1998) Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity 8: 751–759 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

