HER-2 overexpression differentially alters transforming growth factor-beta responses in luminal versus mesenchymal human breast cancer cells
- PMID: 16457687
- PMCID: PMC1410754
- DOI: 10.1186/bcr1343
HER-2 overexpression differentially alters transforming growth factor-beta responses in luminal versus mesenchymal human breast cancer cells
Abstract
Introduction: Amplification of the HER-2 receptor tyrosine kinase has been implicated in the pathogenesis and aggressive behavior of approximately 25% of invasive human breast cancers. Clinical and experimental evidence suggest that aberrant HER-2 signaling contributes to tumor initiation and disease progression. Transforming growth factor beta (TGF-beta) is the dominant factor opposing growth stimulatory factors and early oncogene activation in many tissues, including the mammary gland. Thus, to better understand the mechanisms by which HER-2 overexpression promotes the early stages of breast cancer, we directly assayed the cellular and molecular effects of TGF-beta1 on breast cancer cells in the presence or absence of overexpressed HER-2.
Methods: Cell proliferation assays were used to determine the effect of TGF-beta on the growth of breast cancer cells with normal or high level expression of HER-2. Affymetrix microarrays combined with Northern and western blot analysis were used to monitor the transcriptional responses to exogenous TGF-beta1 in luminal and mesenchymal-like breast cancer cells. The activity of the core TGF-beta signaling pathway was assessed using TGF-beta1 binding assays, phospho-specific Smad antibodies, immunofluorescent staining of Smad and Smad DNA binding assays.
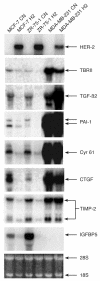
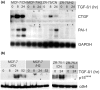
Results: We demonstrate that cells engineered to over-express HER-2 are resistant to the anti-proliferative effect of TGF-beta1. HER-2 overexpression profoundly diminishes the transcriptional responses induced by TGF-beta in the luminal MCF-7 breast cancer cell line and prevents target gene induction by a novel mechanism that does not involve the abrogation of Smad nuclear accumulation, DNA binding or changes in c-myc repression. Conversely, HER-2 overexpression in the context of the mesenchymal MDA-MB-231 breast cell line potentiated the TGF-beta induced pro-invasive and pro-metastatic gene signature.
Conclusion: HER-2 overexpression promotes the growth and malignancy of mammary epithelial cells, in part, by conferring resistance to the growth inhibitory effects of TGF-beta. In contrast, HER-2 and TGF-beta signaling pathways can cooperate to promote especially aggressive disease behavior in the context of a highly invasive breast tumor model.
Figures



Similar articles
-
Overexpression of HER2 (erbB2) in human breast epithelial cells unmasks transforming growth factor beta-induced cell motility.J Biol Chem. 2004 Jun 4;279(23):24505-13. doi: 10.1074/jbc.M400081200. Epub 2004 Mar 24. J Biol Chem. 2004. PMID: 15044465
-
Transforming growth factor-β 1 enhances the invasiveness of breast cancer cells by inducing a Smad2-dependent epithelial-to-mesenchymal transition.Oncol Rep. 2013 Jan;29(1):219-25. doi: 10.3892/or.2012.2111. Epub 2012 Oct 30. Oncol Rep. 2013. PMID: 23129177
-
Transforming growth factor {beta} (TGF-{beta})-Smad target gene protein tyrosine phosphatase receptor type kappa is required for TGF-{beta} function.Mol Cell Biol. 2005 Jun;25(11):4703-15. doi: 10.1128/MCB.25.11.4703-4715.2005. Mol Cell Biol. 2005. PMID: 15899872 Free PMC article.
-
Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer.Biochim Biophys Acta. 2007 Jan;1775(1):21-62. doi: 10.1016/j.bbcan.2006.06.004. Epub 2006 Jul 8. Biochim Biophys Acta. 2007. PMID: 16904831 Review.
-
The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells.J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):169-90. doi: 10.1007/s10911-010-9181-1. Epub 2010 May 15. J Mammary Gland Biol Neoplasia. 2010. PMID: 20467795 Free PMC article. Review.
Cited by
-
Detection of HER-2/neu, c-myc amplification and p53 inactivation by FISH in Egyptian patients with breast cancer.Ger Med Sci. 2009 May 6;7:Doc03. doi: 10.3205/000062. Ger Med Sci. 2009. PMID: 19675743 Free PMC article.
-
Relation between transforming growth factor-β1 expression, its receptor and clinicopathological factors and survival in HER2-negative gastric cancers.Wien Klin Wochenschr. 2011 Nov;123(21-22):668-73. doi: 10.1007/s00508-011-0078-9. Epub 2011 Oct 24. Wien Klin Wochenschr. 2011. PMID: 22015652
-
Key signaling nodes in mammary gland development and cancer: Smad signal integration in epithelial cell plasticity.Breast Cancer Res. 2012 Feb 8;14(1):204. doi: 10.1186/bcr3066. Breast Cancer Res. 2012. PMID: 22315972 Free PMC article. Review.
-
Causal relationships between immune cell traits and HER2 subtypes in breast cancer: a Mendelian randomization study.Discov Oncol. 2025 Aug 5;16(1):1475. doi: 10.1007/s12672-025-03358-6. Discov Oncol. 2025. PMID: 40764893 Free PMC article.
-
Biodistribution and pharmacokinetics of EGFR-targeted thiolated gelatin nanoparticles following systemic administration in pancreatic tumor-bearing mice.Mol Pharm. 2013 May 6;10(5):2031-44. doi: 10.1021/mp400054e. Epub 2013 Apr 16. Mol Pharm. 2013. PMID: 23544877 Free PMC article.
References
-
- King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229:974–976. - PubMed
-
- Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

