Two unrelated putative membrane-bound progestin receptors, progesterone membrane receptor component 1 (PGMRC1) and membrane progestin receptor (mPR) beta, are expressed in the rainbow trout oocyte and exhibit similar ovarian expression patterns
- PMID: 16457725
- PMCID: PMC1373632
- DOI: 10.1186/1477-7827-4-6
Two unrelated putative membrane-bound progestin receptors, progesterone membrane receptor component 1 (PGMRC1) and membrane progestin receptor (mPR) beta, are expressed in the rainbow trout oocyte and exhibit similar ovarian expression patterns
Abstract
Background: In lower vertebrates, steroid-induced oocyte maturation is considered to involve membrane-bound progestin receptors. Two totally distinct classes of putative membrane-bound progestin receptors have been reported in vertebrates. A first class of receptors, now termed progesterone membrane receptor component (PGMRC; subtypes 1 and 2) has been studied since 1996 but never studied in a fish species nor in the oocyte of any animal species. A second class of receptors, termed membrane progestin receptors (mPR; subtypes alpha, beta and gamma), was recently described in vertebrates and implicated in the progestin-initiated induction of oocyte maturation in fish.
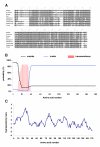
Methods: In the present study, we report the characterization of the full coding sequence of rainbow trout PGMRC1 and mPR beta cDNAs, their tissue distribution, their ovarian expression profiles during oogenesis, their hormonal regulation in the full grown ovary and the in situ localization of PGMRC1 mRNA in the ovary.
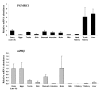
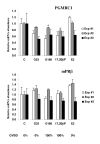
Results: Our results clearly show, for the first time in any animal species, that rainbow trout PGMRC1 mRNA is present in the oocyte and has a strong expression in ovarian tissue. In addition, we show that both mPR beta and PGMRC1, two members of distinct membrane-bound progestin receptor classes, exhibit highly similar ovarian expression profiles during the reproductive cycle with maximum levels during vitellogenesis and a down-expression during late vitellogenesis. In addition, the mRNA abundance of both genes is not increased after in vitro hormonal stimulation of full grown follicles by maturation inducing hormones.
Conclusion: Together, our findings suggest that PGMRC1 is a new possible participant in the progestin-induced oocyte maturation in fish. However, its participation in the process of oocyte maturation, which remains to be confirmed, would occur at post-transcriptional levels.
Figures


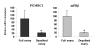

References
-
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

