Pathological pattern of Mdx mice diaphragm correlates with gradual expression of the short utrophin isoform Up71
- PMID: 16457992
- PMCID: PMC1974843
- DOI: 10.1016/j.bbadis.2005.11.006
Pathological pattern of Mdx mice diaphragm correlates with gradual expression of the short utrophin isoform Up71
Abstract
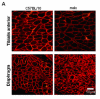
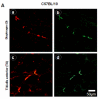
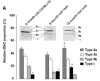
Utrophin gene is transcribed in a large mRNA of 13 kb that codes for a protein of 395 kDa. It shows amino acid identity with dystrophin of up to 73% and is widely expressed in muscle and non-muscle tissues. Up71 is a short utrophin product of the utrophin gene with the same cysteine-rich and C-terminal domains as full-length utrophin (Up395). Using RT-PCR, Western blots analysis, we demonstrated that Up71 is overexpressed in the mdx diaphragm, the most pathological muscle in dystrophin-deficient mdx mice, compared to wild-type C57BL/10 or other mdx skeletal muscles. Subsequently, we demonstrated that this isoform displayed an increased expression level up to 12 months, whereas full-length utrophin (Up395) decreased. In addition, beta-dystroglycan, the transmembrane glycoprotein that anchors the cytoplasmic C-terminal domain of utrophin, showed similar increase expression in mdx diaphragm, as opposed to other components of the dystrophin-associated protein complex (DAPC) such as alpha-dystrobrevin1 and alpha-sarcoglycan. We demonstrated that Up71 and beta-dystroglycan were progressively accumulated along the extrasynaptic region of regenerating clusters in mdx diaphragm. Our data provide novel functional insights into the pathological role of the Up71 isoform in dystrophinopathies.
Figures















Similar articles
-
Effect of beta-dystroglycan processing on utrophin/Dp116 anchorage in normal and mdx mouse Schwann cell membrane.Neuroscience. 2006 Aug 25;141(2):607-620. doi: 10.1016/j.neuroscience.2006.04.043. Epub 2006 Jun 2. Neuroscience. 2006. PMID: 16735092 Free PMC article.
-
Age-related changes in dystrophin-glycoprotein complex and in utrophin are not correlated with intrinsic laryngeal muscles protection in mdx mice.Muscle Nerve. 2011 Dec;44(6):978-80. doi: 10.1002/mus.22235. Muscle Nerve. 2011. PMID: 22102469
-
Differential expression of utrophin-A and -B promoters in the central nervous system (CNS) of normal and dystrophic mdx mice.Brain Pathol. 2010 Mar;20(2):323-42. doi: 10.1111/j.1750-3639.2009.00275.x. Epub 2009 May 22. Brain Pathol. 2010. PMID: 19486009 Free PMC article.
-
What do mouse models of muscular dystrophy tell us about the DAPC and its components?Int J Exp Pathol. 2014 Dec;95(6):365-77. doi: 10.1111/iep.12095. Epub 2014 Sep 30. Int J Exp Pathol. 2014. PMID: 25270874 Free PMC article. Review.
-
Understanding dystrophinopathies: an inventory of the structural and functional consequences of the absence of dystrophin in muscles of the mdx mouse.J Muscle Res Cell Motil. 1999 Oct;20(7):605-25. doi: 10.1023/a:1005545325254. J Muscle Res Cell Motil. 1999. PMID: 10672510 Review. No abstract available.
Cited by
-
Inhibition of muscle fibrosis results in increases in both utrophin levels and the number of revertant myofibers in Duchenne muscular dystrophy.Oncotarget. 2015 Sep 15;6(27):23249-60. doi: 10.18632/oncotarget.4021. Oncotarget. 2015. PMID: 26015394 Free PMC article.
-
Second-generation compound for the modulation of utrophin in the therapy of DMD.Hum Mol Genet. 2015 Aug 1;24(15):4212-24. doi: 10.1093/hmg/ddv154. Epub 2015 May 1. Hum Mol Genet. 2015. PMID: 25935002 Free PMC article.
-
Loss of cIAP1 attenuates soleus muscle pathology and improves diaphragm function in mdx mice.Hum Mol Genet. 2013 Mar 1;22(5):867-78. doi: 10.1093/hmg/dds493. Epub 2012 Nov 25. Hum Mol Genet. 2013. PMID: 23184147 Free PMC article.
-
Dystrophins, utrophins, and associated scaffolding complexes: role in mammalian brain and implications for therapeutic strategies.J Biomed Biotechnol. 2010;2010:849426. doi: 10.1155/2010/849426. Epub 2010 Jun 17. J Biomed Biotechnol. 2010. PMID: 20625423 Free PMC article. Review.
References
-
- Chakkalakal JV, Thompson J, Parks RJ, Jasmin BJ. Molecular, cellular, and pharmacological therapies for duchenne/becker muscular dystrophies. Faseb J. 2005;19:880–91. - PubMed
-
- Ahn AH, Kunkel LM. The structural and functional diversity of dystrophin. Nat Genet. 1993;3:283–91. - PubMed
-
- De Bleecker JL, Engel AG. Expression of cell adhesion molecules in inflammatory myopathies and duchenne dystrophy. J Neuropathol Exp Neurol. 1994;53:369–76. - PubMed
-
- Matsumura K, Campbell KP. Dystrophin-glycoprotein complex: Its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994;17:2–15. - PubMed
-
- Higuchi I, Niiyama T, Fukunaga H, Nakamura K, Nakagawa M, Osame M. Dystrophin-related protein in becker muscular dystrophy. Intern Med. 1994;33:334–6. - PubMed

