Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity
- PMID: 16461338
- PMCID: PMC2118210
- DOI: 10.1084/jem.20051720
Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity
Abstract
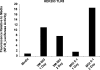
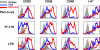
The live attenuated yellow fever vaccine 17D (YF-17D) is one of the most effective vaccines available, with a 65-yr history of use in >400 million people globally. Despite this efficacy, there is presently no information about the immunological mechanisms by which YF-17D acts. Here, we present data that suggest that YF-17D activates multiple Toll-like receptors (TLRs) on dendritic cells (DCs) to elicit a broad spectrum of innate and adaptive immune responses. Specifically, YF-17D activates multiple DC subsets via TLRs 2, 7, 8, and 9 to elicit the proinflammatory cytokines interleukin (IL)-12p40, IL-6, and interferon-alpha. Interestingly, the resulting adaptive immune responses are characterized by a mixed T helper cell (Th)1/Th2 cytokine profile and antigen-specific CD8+ T cells. Furthermore, distinct TLRs appear to differentially control the Th1/Th2 balance; thus, whilst MyD88-deficient mice show a profound impairment of Th1 cytokines, TLR2-deficient mice show greatly enhanced Th1 and Tc1 responses to YF-17D. Together, these data enhance our understanding of the molecular mechanism of action of YF-17D, and highlight the potential of vaccination strategies that use combinations of different TLR ligands to stimulate polyvalent immune responses.
Figures





References
-
- Niedrig, M., M. Lademann, P. Emmerich, and M. Lafrenz. 1999. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop. Med. Int. Health. 4:867–871. - PubMed
-
- Co, M.D., M. Terajima, J. Cruz, F.A. Ennis, and A.L. Rothman. 2002. Human cytotoxic T lymphocyte responses to live attenuated 17D yellow fever vaccine: identification of HLA-B35-restricted CTL epitopes on nonstructural proteins NS1, NS2b, NS3, and the structural protein E. Virology. 293:151–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

