ATM regulates ATR chromatin loading in response to DNA double-strand breaks
- PMID: 16461339
- PMCID: PMC2118201
- DOI: 10.1084/jem.20051923
ATM regulates ATR chromatin loading in response to DNA double-strand breaks
Abstract
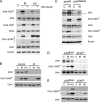
DNA double-strand breaks (DSBs) are among the most deleterious lesions that can challenge genomic integrity. Concomitant to the repair of the breaks, a rapid signaling cascade must be coordinated at the lesion site that leads to the activation of cell cycle checkpoints and/or apoptosis. In this context, ataxia telangiectasia mutated (ATM) and ATM and Rad-3-related (ATR) protein kinases are the earliest signaling molecules that are known to initiate the transduction cascade at damage sites. The current model places ATM and ATR in separate molecular routes that orchestrate distinct pathways of the checkpoint responses. Whereas ATM signals DSBs arising from ionizing radiation (IR) through a Chk2-dependent pathway, ATR is activated in a variety of replication-linked DSBs and leads to activation of the checkpoints in a Chk1 kinase-dependent manner. However, activation of the G2/M checkpoint in response to IR escapes this accepted paradigm because it is dependent on both ATM and ATR but independent of Chk2. Our data provides an explanation for this observation and places ATM activity upstream of ATR recruitment to IR-damaged chromatin. These data provide experimental evidence of an active cross talk between ATM and ATR signaling pathways in response to DNA damage.
Figures




References
-
- Elledge, S.J. 1996. Cell cycle checkpoints: preventing an identity crisis. Science. 274:1664–1672. - PubMed
-
- Rouse, J., and S.P. Jackson. 2002. Interfaces between the detection, signaling, and repair of DNA damage. Science. 297:547–551. - PubMed
-
- Kastan, M.B., and J. Bartek. 2004. Cell-cycle checkpoints and cancer. Nature. 432:316–323. - PubMed
-
- Bartkova, J., Z. Horejsi, K. Koed, A. Kramer, F. Tort, K. Zieger, P. Guldberg, M. Sehested, J.M. Nesland, C. Lukas, et al. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 434:864–870. - PubMed
-
- Gorgoulis, V.G., L.V. Vassiliou, P. Karakaidos, P. Zacharatos, A. Kotsinas, T. Liloglou, M. Venere, R.A. Ditullio Jr., N.G. Kastrinakis, B. Levy, et al. 2005. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 434:907–913. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

