Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity
- PMID: 16461340
- PMCID: PMC2118217
- DOI: 10.1084/jem.20051986
Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity
Abstract
Killer cell lectin-like receptor G1 (KLRG1) is an inhibitory receptor expressed on subsets of natural killer (NK) cells and T cells, for which no endogenous ligands are known. Here, we show that KLRG1 binds three of the classical cadherins (E-, N-, and R-), which are ubiquitously expressed in vertebrates and mediate cell-cell adhesion by homotypic or heterotypic interactions. By expression cloning using the mouse KLRG1 tetramer as a probe, we identified human E-cadherin as a xenogeneic ligand. We also identified a syngeneic interaction between mouse KLRG1 and mouse E-cadherin. Furthermore, we show that KLRG1 binds N- and R-cadherins. Finally, we demonstrate that E-cadherin binding of KLRG1 prevents the lysis of E-cadherin-expressing targets by KLRG1+ NK cells. These results suggest that KLRG1 ligation by E-, N-, or R-cadherins may regulate the cytotoxicity of killer cells to prevent damage to tissues expressing the cadherins.
Figures

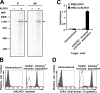

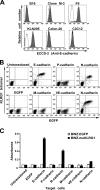
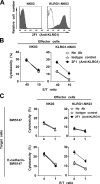
Comment in
-
Cytolytic responses: cadherins put out the fire.J Exp Med. 2006 Feb 20;203(2):261-4. doi: 10.1084/jem.20052559. Epub 2006 Feb 6. J Exp Med. 2006. PMID: 16461342 Free PMC article. Review.
References
-
- Biron, C.A., K.B. Nguyen, G.C. Pien, L.P. Cousens, and T.P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189–220. - PubMed
-
- Lanier, L.L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225–274. - PubMed
-
- Long, E.O. 1999. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 17:875–904. - PubMed
-
- Moretta, L., and A. Moretta. 2004. Killer immunoglobulin-like receptors. Curr. Opin. Immunol. 16:626–633. - PubMed
-
- Yokoyama, W.M. 1998. Natural killer cell receptors. Curr. Opin. Immunol. 10:298–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

