H2-M3-restricted CD8+ T cells are not required for MHC class Ib-restricted immunity against Listeria monocytogenes
- PMID: 16461341
- PMCID: PMC2118191
- DOI: 10.1084/jem.20052256
H2-M3-restricted CD8+ T cells are not required for MHC class Ib-restricted immunity against Listeria monocytogenes
Abstract
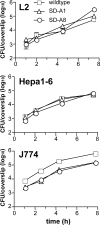
Studies using major histocompatibility complex (MHC)-Ia-deficient mice have shown that MHC-Ib-restricted CD8+ T cells can clear infections caused by intracellular pathogens such as Listeria monocytogenes. M3-restricted CD8+ T cells, which recognize short hydrophobic N-formylated peptides, appear to comprise a substantial portion of the MHC-Ib-restricted T cell response in the mouse model of L. monocytogenes infection. In this study, we isolated formyltransferase (fmt) mutant strains of L. monocytogenes that lacked the ability to add formyl groups to nascent polypeptides. These fmt mutant Listeria strains did not produce antigens that could be recognized by M3-restricted T cells. We showed that immunization of MHC-Ia-deficient mice with fmt mutant Listeria resulted in stimulation of a protective memory response that cleared subsequent challenge with wild-type L. monocytogenes, despite the fact that M3-restricted CD8+ T cells did not proliferate in these mice. These data suggest that M3-restricted T cells are not required for protection against L. monocytogenes and underscore the importance of searching for new antigen-presenting molecules among the large MHC-Ib family of proteins.
Figures






Similar articles
-
Class Ia MHC-deficient BALB/c mice generate CD8+ T cell-mediated protective immunity against Listeria monocytogenes infection.J Immunol. 2003 Jul 1;171(1):291-8. doi: 10.4049/jimmunol.171.1.291. J Immunol. 2003. PMID: 12817010
-
Nonconventional CD8+ T cell responses to Listeria infection in mice lacking MHC class Ia and H2-M3.J Immunol. 2011 Jan 1;186(1):489-98. doi: 10.4049/jimmunol.1002639. Epub 2010 Nov 22. J Immunol. 2011. PMID: 21098224 Free PMC article.
-
Response to Listeria monocytogenes in mice lacking MHC class Ia molecules.J Immunol. 1999 May 1;162(9):5429-36. J Immunol. 1999. PMID: 10228021
-
Role of MHC class Ib molecule, H2-M3 in host immunity against tuberculosis.Vaccine. 2013 Aug 20;31(37):3818-25. doi: 10.1016/j.vaccine.2013.04.005. Epub 2013 Apr 28. Vaccine. 2013. PMID: 23628242 Review.
-
Processing of Listeria monocytogenes antigens and the in vivo T-cell response to bacterial infection.Immunol Rev. 1999 Dec;172:163-9. doi: 10.1111/j.1600-065x.1999.tb01364.x. Immunol Rev. 1999. PMID: 10631945 Review.
Cited by
-
Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity.Crit Rev Immunol. 2006;26(4):317-52. doi: 10.1615/critrevimmunol.v26.i4.30. Crit Rev Immunol. 2006. PMID: 17073557 Free PMC article. Review.
-
Use of the CD107 mobilization assay reveals that cytotoxic T lymphocytes with novel MHC-Ib restriction are activated during Listeria monocytogenes infection.J Immunol Methods. 2007 Dec 1;328(1-2):45-52. doi: 10.1016/j.jim.2007.08.005. Epub 2007 Aug 30. J Immunol Methods. 2007. PMID: 17900608 Free PMC article.
-
The role of MHC class Ib-restricted T cells during infection.Immunogenetics. 2016 Aug;68(8):677-91. doi: 10.1007/s00251-016-0932-z. Epub 2016 Jul 1. Immunogenetics. 2016. PMID: 27368413 Free PMC article. Review.
-
Innate and Adaptive Immune Responses during Listeria monocytogenes Infection.Microbiol Spectr. 2019 May;7(3):10.1128/microbiolspec.gpp3-0065-2019. doi: 10.1128/microbiolspec.GPP3-0065-2019. Microbiol Spectr. 2019. PMID: 31124430 Free PMC article.
-
Recognition of Listeria monocytogenes infection by natural killer cells: Towards a complete picture by experimental studies in rats.Innate Immun. 2023 Aug;29(6):110-121. doi: 10.1177/17534259231178223. Epub 2023 Jun 7. Innate Immun. 2023. PMID: 37285590 Free PMC article. Review.
References
-
- Busch, D.H., K. Kerksiek, and E.G. Pamer. 1999. Processing of Listeria monocytogenes antigens and the in vivo T-cell response to bacterial infection. Immunol. Rev. 172:163–169. - PubMed
-
- Geginat, G., S. Schenk, M. Skoberne, W. Goebel, and H. Hof. 2001. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J. Immunol. 166:1877–1884. - PubMed
-
- Soloski, M.J., M.E. Szperka, A. Davies, and S.L. Wooden. 2000. Host immune response to intracellular bacteria: a role for MHC-linked class-Ib antigen-presenting molecules. Proc. Soc. Exp. Biol. Med. 224:231–239. - PubMed
-
- Forman, J., and K.F. Lindahl. 2002. Listing, Location, Binding Motifs, and Expression of Nonclassical Class I and Related Genes and Molecules. In Current Protocols in Immunology. John Wiley & Sons, Hoboken, NJ. A.1M1–A.1M13. - PubMed
-
- Shawar, S.M., J.M. Vyas, J.R. Rodgers, and R.R. Rich. 1994. Antigen presentation by major histocompatibility complex class Ib molecules. Annu. Rev. Immunol. 12:839–880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

