NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly
- PMID: 16461362
- PMCID: PMC2063671
- DOI: 10.1083/jcb.200510028
NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly
Abstract
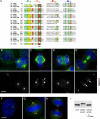
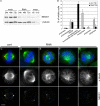
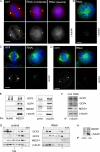
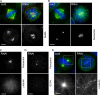
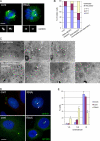
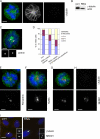
The centrosome is the major microtubule organizing structure in somatic cells. Centrosomal microtubule nucleation depends on the protein gamma-tubulin. In mammals, gamma-tubulin associates with additional proteins into a large complex, the gamma-tubulin ring complex (gammaTuRC). We characterize NEDD1, a centrosomal protein that associates with gammaTuRCs. We show that the majority of gammaTuRCs assemble even after NEDD1 depletion but require NEDD1 for centrosomal targeting. In contrast, NEDD1 can target to the centrosome in the absence of gamma-tubulin. NEDD1-depleted cells show defects in centrosomal microtubule nucleation and form aberrant mitotic spindles with poorly separated poles. Similar spindle defects are obtained by overexpression of a fusion protein of GFP tagged to the carboxy-terminal half of NEDD1, which mediates binding to gammaTuRCs. Further, we show that depletion of NEDD1 inhibits centriole duplication, as does depletion of gamma-tubulin. Our data suggest that centriole duplication requires NEDD1-dependent recruitment of gamma-tubulin to the centrosome.
Figures


References
-
- Andersen, J.S., C.J. Wilkinson, T. Mayor, P. Mortensen, E.A. Nigg, and M. Mann. 2003. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 426:570–574. - PubMed
-
- Barbosa, V., M. Gatt, E. Rebollo, C. Gonzalez, and D.M. Glover. 2003. Drosophila dd4 mutants reveal that gammaTuRC is required to maintain juxtaposed half spindles in spermatocytes. J. Cell Sci. 116:929–941. - PubMed

