Zero mode waveguides for single-molecule spectroscopy on lipid membranes
- PMID: 16461393
- PMCID: PMC1432119
- DOI: 10.1529/biophysj.105.072819
Zero mode waveguides for single-molecule spectroscopy on lipid membranes
Abstract
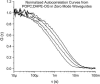
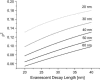
Zero mode waveguides (ZMWs), subwavelength optical nanostructures with dimensions ranging from 50 to 200 nm, have been used to study systems involving ligand-receptor interactions. We show that under proper conditions, lipid membranes will invaginate into the nanostructures, which confine optical excitation to subattoliter volumes. Fluorescence correlation spectroscopy (FCS) was used to characterize the diffusion of fluorescently tagged lipids in liquid-disordered phase 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and gel phase 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) membranes incubated on the nanostructured surface. In contrast to the POPC, DSPC membranes did not appear to enter the structures, suggesting that invagination is dependent on membrane rigidity. Although correlation curves obtained from POPC membranes conformed to previously derived models for diffusion in the evanescent field within the nanostructure, the diffusion constants obtained were systematically lower than expected. The validity of the one-dimensional diffusion model for membrane diffusion is discussed and it is concluded that the erroneous diffusion constants are a result of nontrivial membrane conformation within the ZMWs. Additionally, FCS was used to characterize the fraction of fluorescently labeled tetanus toxin C fragment bound to a ganglioside-populated POPC membrane within the ZMWs. This allowed the determination of the toxin's equilibrium binding constant at a concentration of 500 nM; higher than possible with diffraction-limited FCS. To our knowledge, the results presented here are the first reported for supported lipid bilayers in nanostructured devices. Furthermore, they open the possibility of studying membrane imbedded receptors and proteins at physiological concentrations with single-molecule resolution.
Figures








References
-
- Lipowsky, R. 1995. The morphology of lipid membranes. Curr. Opin. Struct. Biol. 5:531–540. - PubMed
-
- Lipowsky, R. 1995. From bunches of membranes to bundles of strings. Zeitschrift Fur Physik B-Condensed Matter. 97:193–203.
-
- Lipowsky, R., and S. Leibler. 1986. Unbinding transitions of interacting membranes. Phys. Rev. Lett. 56:2541–2544. - PubMed
-
- Mutz, M., and W. Helfrich. 1989. Unbinding transition of a biological model membrane. Phys. Rev. Lett. 62:2881–2884. - PubMed
-
- Lipowsky, R. 1994. Discontinuous unbinding transitions of flexible membranes. Journal De Physique Ii. 4:1755–1762.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

