Using plasma topotecan pharmacokinetics to estimate topotecan exposure in cerebrospinal fluid of children with medulloblastoma
- PMID: 16461424
- PMCID: PMC1871944
- DOI: 10.1215/15228517-2005-004
Using plasma topotecan pharmacokinetics to estimate topotecan exposure in cerebrospinal fluid of children with medulloblastoma
Abstract
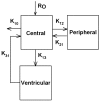
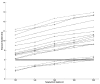
The purpose of this study was to estimate ventricular cerebrospinal fluid (vCSF) topotecan lactone (TPT) exposures in pediatric medulloblastoma patients from plasma concentration-time data by using a pharmacokinetic (PK) model. We studied children with high-risk medulloblastoma who received pharmacokinetically guided TPT (target plasma area under the concentration-time curve [AUC], 120-160 ng/ml-h) and obtained serial vCSF samples to assess TPT exposure. Population pharmacokinetic parameters were determined by using linear mixed-effects modeling via the two-stage approach. We simulated TPT vCSF exposure duration at plasma TPT AUC values of 120 to 200 ng/ml-h and determined percentages of studies meeting or exceeding the vCSF exposure duration threshold (EDT) of 1 ng/ml for 8 h. We then used bootstrap methods to estimate variability in vCSF EDT. Eighteen PK studies were conducted in six patients (median age, 4.8 years). In these patients, seven of nine studies attaining target plasma TPT AUC achieved the vCSF EDT. Given a plasma TPT AUC of 120 ng/ml-h, the median percentage of results meeting or exceeding EDT was 78% (95% CI, 61%-100%). One patient (four studies) with tumor blockage of CSF flow, which can alter CSF pharmacokinetics, was removed, and the bootstrap analysis was repeated. In this subset, a median 93% (95% CI, 79%-100%) of studies achieved vCSF EDT. Increasing plasma TPT AUC values resulted in increased ability to achieve vCSF EDT. We demonstrated that a plasma PK model could estimate vCSF TPT concentrations. Further, our results indicate that the TPT vCSF EDT can be achieved in more than 80% of studies targeted to a plasma TPT AUC of 120 ng/ml-h.
Figures
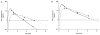
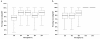
Similar articles
-
A four-hour topotecan infusion achieves cytotoxic exposure throughout the neuraxis in the nonhuman primate model: implications for treatment of children with metastatic medulloblastoma.Clin Cancer Res. 1998 Oct;4(10):2537-44. Clin Cancer Res. 1998. PMID: 9796988
-
Results of a phase II upfront window of pharmacokinetically guided topotecan in high-risk medulloblastoma and supratentorial primitive neuroectodermal tumor.J Clin Oncol. 2004 Aug 15;22(16):3357-65. doi: 10.1200/JCO.2004.10.103. J Clin Oncol. 2004. PMID: 15310781 Clinical Trial.
-
Phenytoin alters the disposition of topotecan and N-desmethyl topotecan in a patient with medulloblastoma.Clin Cancer Res. 1998 Mar;4(3):783-9. Clin Cancer Res. 1998. PMID: 9533548
-
Pharmacokinetically guided administration of chemotherapeutic agents.Clin Pharmacokinet. 2000 Nov;39(5):345-67. doi: 10.2165/00003088-200039050-00004. Clin Pharmacokinet. 2000. PMID: 11108434 Review.
-
Deriving therapies for children with primary CNS tumors using pharmacokinetic modeling and simulation of cerebral microdialysis data.Eur J Pharm Sci. 2014 Jun 16;57:41-7. doi: 10.1016/j.ejps.2013.11.010. Epub 2013 Nov 20. Eur J Pharm Sci. 2014. PMID: 24269626 Free PMC article. Review.
Cited by
-
A phase-1 pharmacokinetic optimal dosing study of intraventricular topotecan for children with neoplastic meningitis: a Pediatric Brain Tumor Consortium study.Pediatr Blood Cancer. 2013 Apr;60(4):627-32. doi: 10.1002/pbc.24309. Epub 2012 Sep 21. Pediatr Blood Cancer. 2013. PMID: 23002039 Free PMC article. Clinical Trial.
-
Ocular Salvage and Vision Preservation Using a Topotecan-Based Regimen for Advanced Intraocular Retinoblastoma.J Clin Oncol. 2017 Jan;35(1):72-77. doi: 10.1200/JCO.2016.69.2996. Epub 2016 Oct 31. J Clin Oncol. 2017. PMID: 28034080 Free PMC article.
-
Population pharmacokinetics and pharmacodynamics for treatment optimization in clinical oncology.Clin Pharmacokinet. 2008;47(8):487-513. doi: 10.2165/00003088-200847080-00001. Clin Pharmacokinet. 2008. PMID: 18611060 Review.
-
Topotecan enhances immune clearance of gliomas.Cancer Immunol Immunother. 2009 Feb;58(2):259-70. doi: 10.1007/s00262-008-0550-1. Epub 2008 Jul 2. Cancer Immunol Immunother. 2009. PMID: 18594817 Free PMC article.
-
Phenothiazines inhibit hepatitis C virus entry, likely by increasing the fluidity of cholesterol-rich membranes.Antimicrob Agents Chemother. 2013 Jun;57(6):2571-81. doi: 10.1128/AAC.02593-12. Epub 2013 Mar 25. Antimicrob Agents Chemother. 2013. PMID: 23529728 Free PMC article.
References
-
- Baker SD, Heideman RL, Crom WR, Kuttesch JF, Gajjar A, Stewart CF. Cerebrospinal fluid pharmacokinetics and penetration of continuous infusion topotecan in children with central nervous system tumors. Cancer Chemother Pharmacol. 1996;37:195–202. - PubMed
-
- Blaney SM, Cole DE, Balis FM, Godwin K, Poplack DG. Plasma and cerebrospinal fluid pharmacokinetic study of topotecan in nonhuman primates. Cancer Res. 1993;53:725–727. - PubMed
-
- D’Argenio, D.Z., and Schumitzky, A. (1990) ADAPT II User’s Guide, 1st ed. Los Angeles: Biomedical Simulations Resource, University of Southern California.
-
- Ellison DW, Clifford SC, Gajjar A, Gilbertson RJ. What’s new in neuro-oncology? Recent advances in medulloblastoma. Eur J Paediatr Neurol. 2003;7:53–66. - PubMed
-
- Gajjar AJ, Kirstein MN, Santana VM, Furman WL, Thompson S, Houghton PJ, Stewart CF. Concomitant dexamethasone (DXM) increases topotecan (TPT) clearance in children. Proc Am Assoc Cancer Res. 2001;42:653. (abstract)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

