Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis
- PMID: 16461582
- PMCID: PMC1383645
- DOI: 10.1105/tpc.105.037978
Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis
Abstract
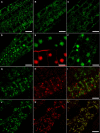
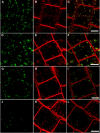
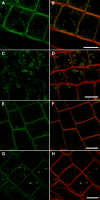
In eukaryotic cells, compartments of the highly dynamic endomembrane system are acidified to varying degrees by the activity of vacuolar H(+)-ATPases (V-ATPases). In the Arabidopsis thaliana genome, most V-ATPase subunits are encoded by small gene families, thus offering potential for a multitude of enzyme complexes with different kinetic properties and localizations. We have determined the subcellular localization of the three Arabidopsis isoforms of the membrane-integral V-ATPase subunit VHA-a. Colocalization experiments as well as immunogold labeling showed that VHA-a1 is preferentially found in the trans-Golgi network (TGN), the main sorting compartment of the secretory pathway. Uptake experiments with the endocytic tracer FM4-64 revealed rapid colocalization with VHA-a1, indicating that the TGN may act as an early endosomal compartment. Concanamycin A, a specific V-ATPase inhibitor, blocks the endocytic transport of FM4-64 to the tonoplast, causes the accumulation of FM4-64 together with newly synthesized plasma membrane proteins, and interferes with the formation of brefeldin A compartments. Furthermore, nascent cell plates are rapidly stained by FM4-64, indicating that endocytosed material is redirected into the secretory flow after reaching the TGN. Together, our results suggest the convergence of the early endocytic and secretory trafficking pathways in the TGN.
Figures




References
-
- Belanger, K.D., and Quatrano, R.S. (2000). Membrane recycling occurs during asymmetric tip growth and cell plate formation in Fucus distichus zygotes. Protoplasma 212 24–37.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

