Pure-culture growth of fermentative bacteria, facilitated by H2 removal: bioenergetics and H2 production
- PMID: 16461652
- PMCID: PMC1392933
- DOI: 10.1128/AEM.72.2.1079-1085.2006
Pure-culture growth of fermentative bacteria, facilitated by H2 removal: bioenergetics and H2 production
Abstract
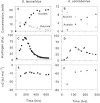
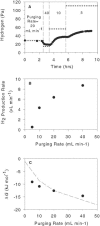
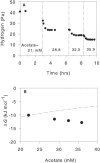
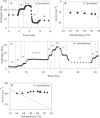
We used an H2-purging culture vessel to replace an H2-consuming syntrophic partner, allowing the growth of pure cultures of Syntrophothermus lipocalidus on butyrate and Aminobacterium colombiense on alanine. By decoupling the syntrophic association, it was possible to manipulate and monitor the single organism's growth environment and determine the change in Gibbs free energy yield (DeltaG) in response to changes in the concentrations of reactants and products, the purging rate, and the temperature. In each of these situations, H2 production changed such that DeltaG remained nearly constant for each organism (-11.1 +/- 1.4 kJ mol butyrate(-1) for S. lipocalidus and -58.2 +/- 1.0 kJ mol alanine(-1) for A. colombiense). The cellular maintenance energy, determined from the DeltaG value and the hydrogen production rate at the point where the cell number was constant, was 4.6 x 10(-13) kJ cell(-1) day(-1) for S. lipocalidus at 55 degrees C and 6.2 x 10(-13) kJ cell(-1) day(-1) for A. colombiense at 37 degrees C. S. lipocalidus, in particular, seems adapted to thrive under conditions of low energy availability.
Figures
References
-
- Baena, S., M. L. Fardeau, M. Labat, B. Ollivier, P. Thomas, J. L. Garcia, and B. K. C. Patel. 1998. Aminobacterium colombiense gen. nov. sp. nov., an amino acid-degrading anaerobe isolated from anaerobic sludge. Anaerobe 4:241-250. - PubMed
-
- Chong, S. C., Y. Liu, M. Cummins, D. L. Valentine, and D. R. Boone. 2002. Methanogenium marinum sp. nov., a H2-using methanogen from Skan Bay, Alaska, and kinetics of H2 utilization. Antonie Leeuwenhoek 81:263-270. - PubMed
-
- Conrad, R., and B. Wetter. 1990. Influence of temperature on energetics of hydrogen metabolism in homoacetogenic, methanogenic, and other anaerobic bacteria. Arch. Microbiol. 155:94-98.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

